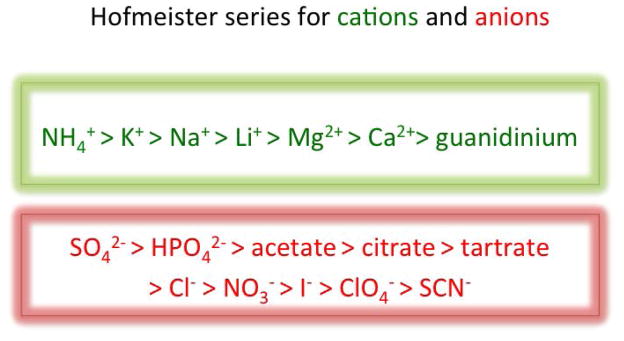
Figure 1.
The Hofmeister series. In 1888 Franz Hofmeister described the ranking of salt solutions for efficacy in precipitating serum globulins (81). By comparing cations paired with the same anion and vice versa he elegantly dissected the effects of the individual ions and ranked them as illustrated in the series. Each series describes a spectrum from the first ion (left), which most decreases globulin solubility (“salting out”) to the last ion (right), which most increases it (“salting in”). Anionic effects tend to be stronger than the cationic ones. The series has also been called lyotropic and the left-hand extreme kosmotropic, whereas the righ-hand extreme is commonly referred to as chaotropic. Those terms are derived from a now much revised view of how the ions work. Thiocynate, the most chaotropic of the anions, is often used in the chaotrope-based avidity assay. Certain non-ionic molecules, e.g., urea, share some properties of the chaotropic ions and have also been used in the assay. The old theory suggested that the effect was general and attributable to how the ions interact with water: the propensity of water molecules to form shells around macromolecules would be diminished by the chaotrope. Part of the binding energy for two interacting protein molecules is explained by how the macromolecular interaction reduces the sizes of those combined water shells. Therefore the binding energy would be unfavorably affected by the chaotrope. The newer insights in Hofmeister solvation science suggest less general mechanisms: instead the solvation effects on proteins partly stem from ionic interactions with the peptidic backbone and the side chains, the latter in particular making the susceptibility of each antibody-antigen complex unique, like the amino-acid composition of the parts contributing to the epitope and the paratope. Already the old view suggested variation in chaotrope resistance according to the relative contributions to the paratope-epitope binding of van der Waals or hydrophobic versus polar interactions. But the new insights further undermine the theoretical basis for using chaotropes to measure binding strength.