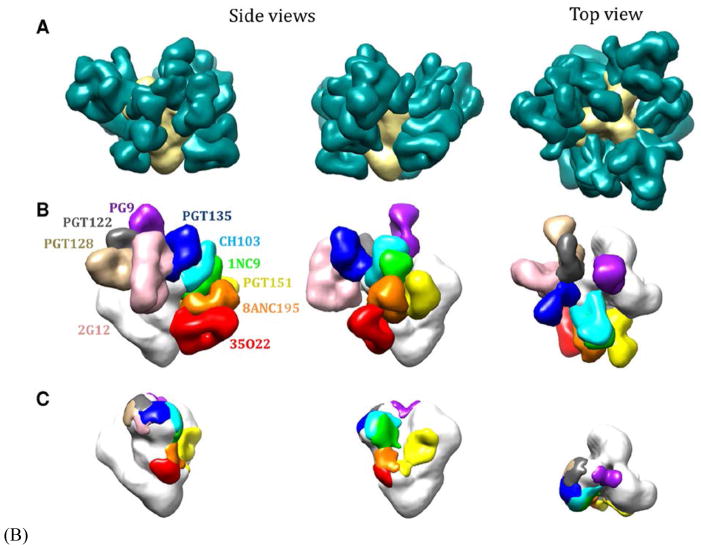
Figure 2. Antibodies (Fabs) bound to neutralization epitopes of an HIV-1 Env trimer.
(A) The Fabs (green) directed to various known neutralization epitopes on each of the three Env protomers nearly cover the trimer (beige). (B) Fabs directed to different epitopes are shown in different colors. Most antibodies bind with a stoichiometry of up three paratopes per trimer; some directed to quaternary-structural epitopes at the trimer apex (PG9) bind to only one epitope per trimer; but here only one Fab of each antibody is represented, thereby revealing the unoccupied surfaces on the other protomers (white). (C) The unoccupied epitopes, corresponding to those occupied by the Fabs in B, are shown in the same colors as the corresponding Fabs in B. The figure shows simultaneous binding of multiple paratopes, a situation that resembles the binding of polyclonal antibodies in a serum although in some sera antibodies to a single epitope dominate. Because the antibodies will have varying affinities and unknown concentrations, only the average off-rate constant can be assessed by kinetic techniques. Furthermore, the binding of one Fab may positively or negatively affect the binding of another (93). The images are reproduced from Derking et al. PLoSPath 2015 11: e1004767 (93).