Abstract
An ultra fast and unbiased method that uses salicylic acid coated magnetic nanoparticles (SAMNPs) and magnetophoretic chromatography is developed to extract chromatin associated RNAs (CARs). The SAMNPs were first used for enriching cells from the cell culture media and further used for capturing chromatin after cells were lysed. The formed SAMNPs-chromatin complexes were transferred to a viscous polyethylene glycol (PEG) solution stored in a 200-μL pipette tip. Due to the difference in viscosities, a bi-layer liquid was formed inside the pipette tip. The SAMNPs-chromatin complexes were separated from the free SAMNPs and free RNA-SAMNPs complexes by applying an external magnetic field. The CARs were further extracted from the SAMNP-chromatin complexes directly. The extracted CARs were reverse transcribed as cDNA and further characterized by real-time qPCR. The total assay time taken for cell separation, chromatin purification and chromatin associated RNAs extraction can be accomplished in less than 2h.
Keywords: Unbiased preparation of chromatin associated RNAs, Magnetic nanoparticle, Magnetophoretic chromatography, Heterochromatin, soluble chromatin
1. Introduction
Chromatin associated RNAs (CARs) are an integral component of chromatin that has diverse architectural functions inside the nucleus. They are found to associate with heterochromatin [1], euchromatin [2], and nuclear matrix [3]. Purification of CARs is extremely important for downstream applications such as transcriptomic analyses. Conventionally, CARs are isolated from soluble chromatin, which is collected from different fractions in a sucrose gradient after overnight centrifugation. The soluble chromatin was released from isolated nuclei by MNase digestion. The total assay time including nuclei preparation, chromatin purification and RNA isolation is about 16 h. The RNA isolated from soluble chromatin-containing fractions might not fully represent the CARs, since RNAs are also associated with insoluble heterochromatin fragments, for example, repetitive RNAs may form more complex and less soluble structures with heterochromatin [2]. Moreover, compared to mRNA, the half live of CARs vary over a wide range with some less than 2 h, for example the paraspeckle RNA Neat1 [4]. Although the sequences of CARs extracted by the conventional method are systematically identified at the global level using high-throughput genomic platforms [5], the study may have some bias due to the fact that some RNAs are resistant to extraction and some RNAs are degraded during the long handling process. Thus, developing an ultrafast and unbiased method for the extraction of CARs is essential to ensure the accuracy of transcriptomic analyses.
One of the most important and time-consuming steps during CAR extraction is the isolation of native chromatin. In contrast to the conventional method that requires high speed centrifugation for a prolonged period of time to purify soluble chromatin, in our previous studies, we developed methods using magnetic nanoparticles, for example salicylic acid coated magnetic nanoparticles (SAMNPs), for a rapid, unbiased and cost-effective method for the purification of chromatin [6, 7]. When free RNA (most of these are rRNAs and mRNAs) and chromatin were released from lysed mammalian cells, these nucleic acids were non-specifically bound to SAMNPs forming free RNA-SAMNPs, chromatin-SAMNPs complexes. The complexes and free magnetic nanoparticles were magnetically separated from the sample solution. If extraction of CARs is desired, both the free RNA-SAMNPs complexes and the free SAMNPs must be removed before chromatin-SAMNPs complexes were submitted for CAR extraction.
This problem can be addressed by adapting magnetophoretic chromatography using a liquid-type filter [8, 9]. A solution containing the free SAMNPs, free RNA-SAMNPs complex and chromatin-SAMNPs complex are loaded onto the surface of a viscous PEG solution in a 200-μl pipette tip to form two separated liquid layers due to differences in viscosities. When an external magnetic field is placed below the tip, only the chromatin-SAMNPs complexes pass through the interface and reach the bottom of the tip; both the free RNA-SAMNPs complexes and the free SAMNPs are trapped at the interface between the solutions. The magnetophoretic chromatography can be achieved in 10 min. The collected chromatin-SAMNPs complexes could be directly used for CARs extraction.
In this study, we present a rapid and unbiased method to extract chromatin associated RNAs by introducing magnetophoretic chromatography to purify chromatin using SAMNPs. SAMNPs have dual functions; first they can be used to enrich mammalian cells from the culture media and further used for capturing chromatin from the lysed cells. Magnetophoretic chromatography was applied to isolate the chromatin-SAMNPs complex from both free SAMNPs and free RNA-SAMNPs complexes. The collected chromatin-SAMNPs complexes were used for CAR extraction. The total assay time including cell and chromatin purification and CARs extraction can be completed within 2h.
2. Experimental
2.1 Reagents and material
Sodium dodecyl sulfate (SDS), phenylmethylsulfonyl fluoride (PMSF), isopropanol, ethylenediaminetetraacetic acid disodium salt (EDTA), polyethylene glycol (PEG) and ethylene glycol tetraacetic acid (EGTA) were obtained from Sigma (St. Louis, MO). Protease Inhibitor Cocktail was purchased from New England Biolabs. PCR probes were synthesized by Integrated DNA Technologies (IDT, USA). Hydrophilic SAMNPs were prepared according per previously reported method [10]. The concentration of SAMNPSs was adjusted to be 10 mg/mL.
2.2 Chromatin isolation and magnetophoretic chromatography
The SAMNPs were added into the cell culture media containing trypsinized MCF7 cells and incubated for 5 min at room temperature. The cell-SAMNPs complexes were magnetically separated and lysed in buffer containing 250 mM SDS, 1 mM EDTA, 1 mM PMSF, 0.5 mM EGTA and 2% protease inhibitor Cocktail [11, 12]. The complexes were pipetted up and down slowly 20 times with a 200-uL pipette tip and further incubated for 10 min. Isopropanol was added to the suspension to form nucleic acids-SAMNPs complexes and incubated for another 10 min on ice. The mixture containing chromatin-SAMNPs, mRNA-SAMNPs complexes and free SAMNPs was separated from the solution by an external magnetic field (SuperMag Multitube Separator), and then re-dispersed in 50-μl 1× PBS. The PBS buffer containing all SAMNPs was transferred onto the 200-μl pipette tip containing 80 μL of a 25 wt% PEG solution. The chromatin-SAMNPs complexes were separated from the free SAMNPs and free RNA-SAMNPs complexes by magnetophoretic chromatography for about 10 min.
2.3 CARs extraction
The collected chromatin-SAMNPs complexes were directly submitted for CARs extraction using E.Z.N.A. Total RNA Kit I (OMEGA bio-tek). In a parallel study, CARs were extracted from isolated nuclei (Nuclei Isolation Kit, Sigma-Aldrich). The concentration and purity of CARs was determined using a Nanodrop spectrophotometer. The yield of CARs was determined as OD260× 40 ng/μl × volume of sample volume (μl). Each extraction was repeated 3 times independently. The CARs were aliquoted into two parts; one was directly used for agarose gel electrophoresis and the other was used for preparing cDNA using the iScript cDNA synthesis kit (Bio-Rad).
2.4 Quantitative (real-time) PCR (qPCR)
The qPCR reactions were performed to validate the enrichment of a subset of frequently reported CARs, and beta-actin mRNA was used as a negative control. The qPCR quantification was performed using the standard curve method with FastStart Universal SYBR Green (Roche) using the LightCycler 96 system (Roche). The primer sequences used in various qPCR reactions are provided in Table 1 [13]. All qPCR reactions were repeated 3 times and the inner control of beta-actin were performed with every qPCR assay. No amplification of the signal was observed when water was added instead of cDNA samples. The relative level of each CAR was calculated compared to the beta-actin mRNA both in the magnetophoretic chromatography purified CARs and in isolated nuclei. Specifically, the cycle threshold (Ct) was defined as the number of cycles required for the fluorescent signal to pass above the baseline established for background by PCR. The 2−ΔΔCt method was used to evaluate the relative expression of CARs. ΔCt was calculated by subtracting the Ct values of each CAR from the Ct values of beta-actin. ΔΔCt was calculated by subtracting ΔCt of each CAR from the corresponding ΔCt of the CAR gained from nuclei, and fold-change of each CAR was determined by the equation 2−ΔΔCt.
Table 1. Nucleotide sequence of primers for qPCR.
| Name | Primer sequence |
|---|---|
| HOTAIR-F | 5′-CAGTGGGGAACTCTGACTCG-3′ |
| HOTAIR-R | 5′-GTGCCTGGTGCTCTCTTACC-3′ |
| NEAT1-F | 5′-TGGCTAGCTCAGGGCTTCAG-3′ |
| NEAT1-R | 5′-TCTCCTTGCCAAGCTTCCTTC-3′ |
| HULC-F | 5′-TCATGATGGAATTGGAGCCTT-3′ |
| HULC-R | 5′-CTCTTCCTGGCTTGCAGATTG-3′ |
| MALAT1-F | 5′-TAGGAAGACAGCAGCAGACAGG-3′ |
| MALAT1-R | 5′-TTGCTCGCTTGCTCCTCAGT-3′ |
| MEG3-F | 5′-GCCAAGCTTCTTGAAAGGCC-3′ |
| MEG3-R | 5′-TTCCACGGAGTAGAGCGAGTC-3′ |
| β-actin-F | 5′-AGCGAGCATCCCCCAAAGTT-3′ |
| β-actin-R | 5′-GGGCACGAAGGCTCATCATT-3′ |
3. Results and discussion
Fig. 1a shows transmission electron microscopy (TEM) images of SAMNPs. The average size of SAMNPs was measured as 10 nm with narrow size distributions. The SAMNPs are monodispersed in water with a hydrodynamic diameter of approximately 122.7 nm and a zeta potential of 38.1 mV [10]. When added into buffers such as cell culture media containing amounts of salts (6.4 g/l NaCl, 3.7 g/l NaHCO3, 0.4 g/l KCl, 0.2 g/l CaCl2 etc), the ions perturb the surface charge of SAMNPs. The SAMNPs form clusters and non-specifically attach to the cell membrane of MCF7 cells, as shown in Fig. 1b.
Fig. 1.
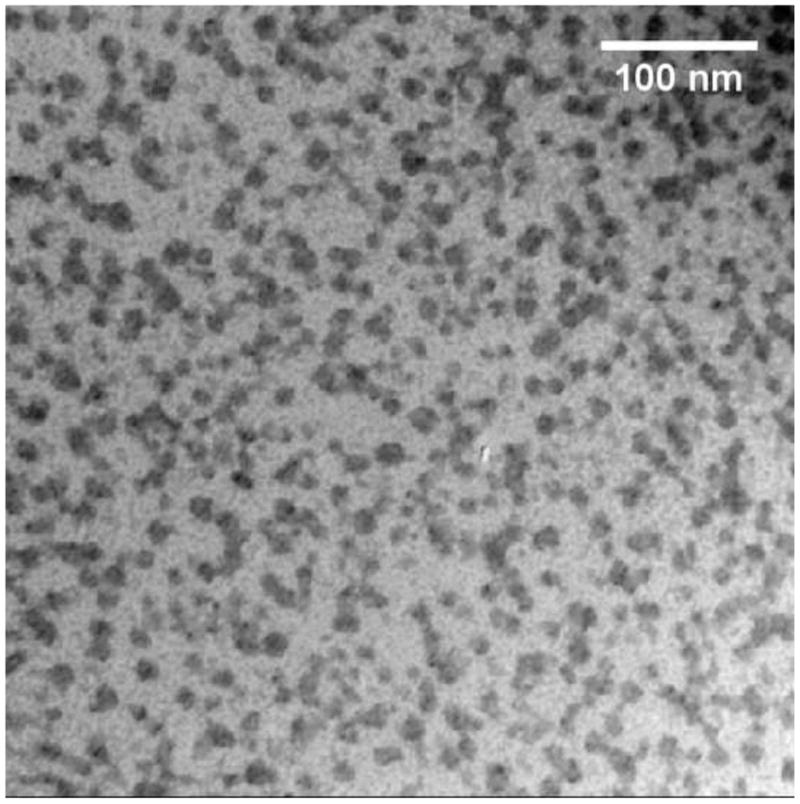
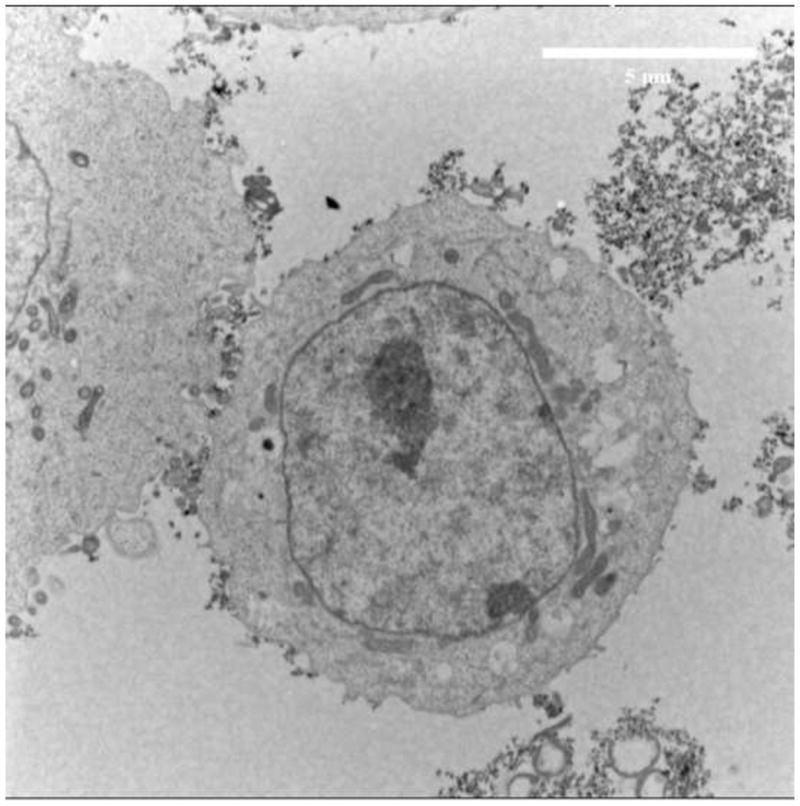
TEM images of (a) SAMNPs and (b) MCF7 cells with SAMNPs. SAMNPs were monodispersed in water and formed clusters when interacting with MCF7 cells in cell culture media.
The formed cell-SAMNPs complexes and free SAMNPs are separated under an external magnetic field. Compared to the free SAMNPs, the cell-SAMNPs are separated more efficiently since the magnetic force exerted on the magnetic particles is proportional to their apparent volume [9]. The small size of SAMNPs helps to retain the superparamagnetic properties [14]. Thus, each SAMNPs cluster consists of a few hundred 10-nm iron oxide nanoparticles, which still possess paramagnetic properties with even higher separation efficiency.
The mixture of cell-SAMNPs complexes and free SAMNPs was magnetically separated from the cell culture media using a permanent magnet (SuperMag Multitube Separator), and then re-suspended in lysis buffer. Nucleic acids, including free RNA and chromatin, were released when the cell membrane and nuclear membrane were dissolved in the lysis buffer. The SAMNPs clusters released from the cell membrane and free SAMNPs were re-dispersed in the lysis buffer and further interacted with the released nucleic acids. Adding chaotropic agents, such as isopropanol, assists nucleic acids to wrap around SAMNPs and cause co-aggregation. Thus, a mixture of free RNA-SAMNPs, chromatin-SAMNPs complexes as well as free SAMNPs was formed and magnetically separated from the lysis buffer with an external magnetic field, and then re-dispersed in 50 μl of 1× PBS for subsequent magnetophoretic chromatography, Fig. 2a.
Fig. 2.
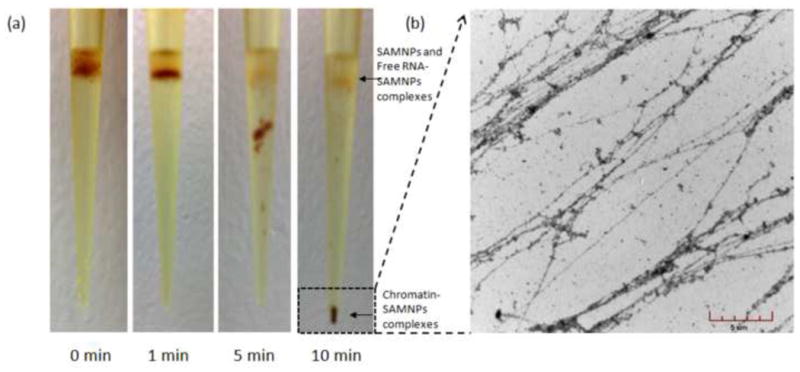
Optical images of the pipette tip during magnetophoretic chromatography of chromatin-SAMNPs in 25% (m/v) PEG solution (a). In the experiment time scale, the chromatin-SAMNPs complexes were isolated at the bottom of the tip; while the free RNA-SAMNPs and free SAMNPs were kept at the interphase of the two solutions. TEM images of chromatin released from the chromatin-SAMNPs complexes in PBS solution (b).
Conventionally, extraction of chromatin associated RNAs utilizes three experimental steps: i) nuclei isolation; ii) soluble chromatin purification; iii) RNA extraction from the collected soluble chromatin. However, both nuclei isolation and the soluble chromatin purification processes are tedious, time consuming and result in significant sample loss. Moreover, the CARs are extracted only from soluble chromatin and RNAs associated with heterochromatin are lost during conventional CARs extraction. And due to short half lives of RNA, CARs degrade during the prolonged extraction process causing a bias for the full sequence analysis of CARs. In contrast, chromatin isolation by the established magnetic isolation process is fast and convenient [6], although there is still the issue of free RNA adsorbing to the magnetic nanoparticle surface, which could introduce artifacts for down-stream sequence analysis of CARs. Thus, it is essential to remove free RNA before transcriptomic analysis of CARs. Most of the free RNAs are mRNA and rRNA in the cytoplasm. Though high temperature could degrade the free RNAs without sacrificing DNA quality and quantity [15], the CARs degrade as well. Thus, it is necessary to develop a fast, simple and cost-effective method to remove free RNA without causing degradation of CARs.
This issue was addressed by adapting magnetophoretic chromatography using a liquid-type filter [8, 9]. Before magnetophoretic separation, all of the magnetic objects were dispersed uniformly in the upper layer of liquid in the pipette tip. When a permanent magnet was placed under the pipette tip, the chromatin-SAMNPs complexes, the free RNA-SAMNPs complexes and free SAMNPs were attracted toward the magnetic pole. Multiple forces affect these complexes through the PEG solution, such as the magnetic force (Fm), gravitational force (Fg), buoyant force (Fb) and drag force (Fd). Fm exerted on an object is proportional to its apparent volume; Fb is proportional to the surface area of the object; Fd is proportional to the solution viscosity, which is directly associated with the concentration of PEG solution [9]. Compared to the free RNA-SAMNPs complexes and the free SAMNPs, the chromatin-SAMNPs complexes are much greater in size due to the large size of chromatin. Thus, the chromatin-SAMNPs complexes have a higher probability of passing through the PEG solution than the free RNA-SAMNPs and the free SAMNPs. To let the chromatin-SAMNPs complexes pass through and keep both the free RNA-SAMNPs complexes and the free SAMNPs at the interface between the PBS solution and PEG solution, the concentration of PEG solution was optimized and determined at 25% (m/v). As shown in Fig. 2a, the chromatin-SAMNPs complexes were well separated from the free RNA-SAMNPs complexes and the free SAMNPs in the experimental time scale (10 min). Only the chromatin-SAMNPs complexes can reach the bottom of the pipette tip after the magnetophoretic chromatography process; and the free RNAs-SAMNPs complexes are trapped at the interface between the solutions.
Due to the reversible process of chromatin and SAMNPs interaction, removing the free SAMNPs from the collected chromatin-SAMNPs complexes would increase the released chromatin in solution. In a compared study, 10 μg of chromatin was extracted from 1 million MCF7 cells using our previously reported method [6]; while 14 μg of chromatin was extracted from the same amount of MCF7 cells using the magnetophoretic chromatography method. The extracted chromatin was visualized by TEM, as shown in Fig. 2b.
The chromatin-SAMNPs complexes were collected after magnetophoretic chromatography and submitted for CARs extraction using a commercial kit. The extraction was completed within 45 min. In a parallel study, CARs were extracted from the isolated nuclei using the same kit. The purity of CARs was determined by OD260/OD280 with a value of 2.05, which indicated there was negligible genomic DNA contamination. The quantity of extracted CARs from the nuclear fraction and the magnetophoretic chromatography collected chromatin were determined as 21.9±0.87 μg and 19.9±1.03 μg, respectively (as shown in Fig. 3a). We used the coefficient of variation (CV), which is defined as the ratio of the standard deviation to the mean, to estimate the reproducibility of the CARs extraction method. The CV with a low value as 5.2% implies that the method proposed is highly reproducible. Using the same amount of MCF7 cells as the starting material, the developed method recovered 90.9±4% RNAs compared to the nuclear fraction. The loss of RNAs could result from removing the free RNAs during the magnetophoretic chromatography process. Agarose gel electrophoresis analysis of the isolated CARs indicated that the major RNAs were rRNA due to rRNA-tree formed at the nucleoli [16].
Fig. 3.
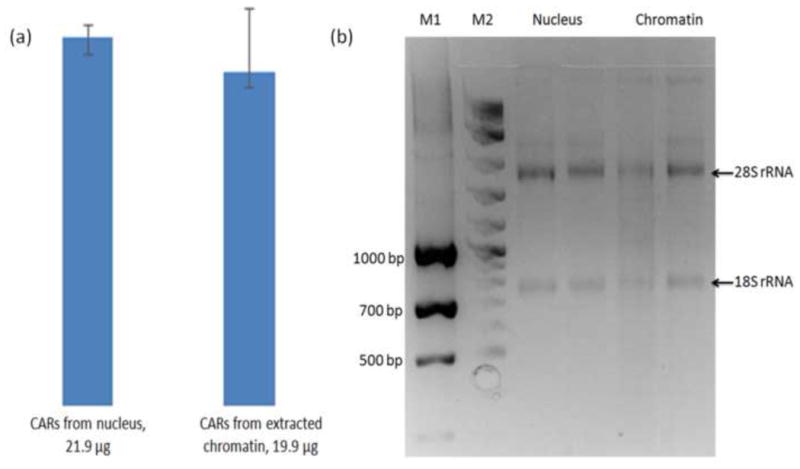
(a) Quantity of CARs extracted from isolated nucleus and magnetophoretic chromatography collected chromatin from 4 million cells. (b) Agarose gel electrophoresis analysis of CARs on a 2% RNase denatured gel.
The quality of CARs was evaluated by real-time PCR (qPCR). The fold change of each CAR was calculated according to the CARs extracted from nuclei, as shown in Fig. 4. The level of β-actin in the extracted CARs, as an internal control, was 5 times lower in the magnetophoretic chromatography collected chromatin compared to CARs extracted from nuclei. As expected, the CARs (MEG3, Neat1, HULC and MALAT1) were 3-8 times higher in the magnetophoretic chromatography collected chromatin than in the nuclear fraction. This is due to the removal of free RNAs unbound to chromatin during the magnetophoretic chromatography process. Interesting HOTAIR was decreased in the collected chromatin, possibly because HOTAIR is primarily located in the cytoplasm of carcinoma cells [17].
Fig. 4.
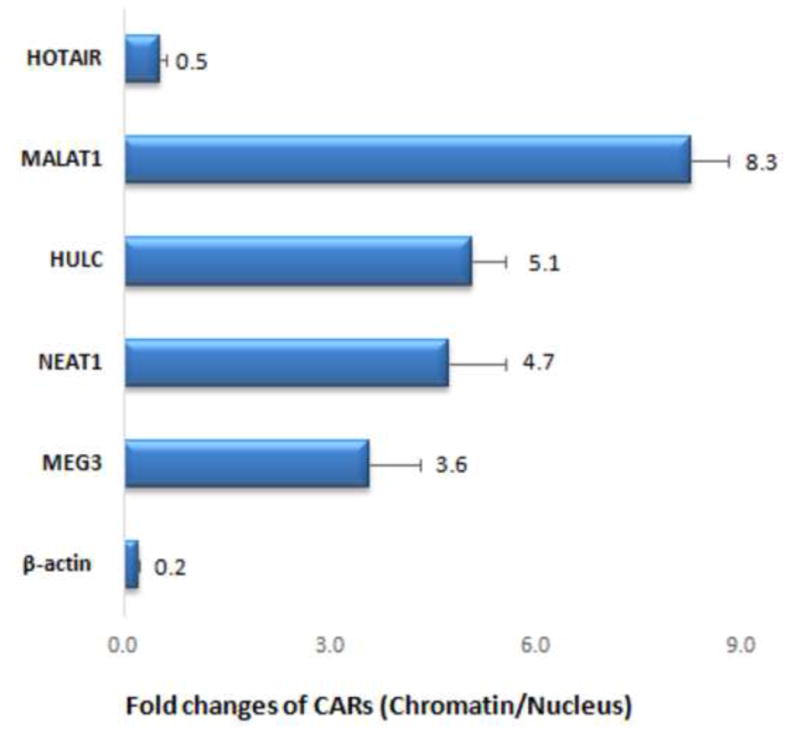
The fold change of CARs extracted from magnetophoretic chromatography collected chromatin compared to that from isolated nucleus. The CARs (MEG3, Neat1, HULC and MALAT1) were 3-8 times higher in the magnetophoretic chromatography collected chromatin than in the nucleus, while β-actin and HOTAIR was lower.
4. Conclusions
In conclusion, using cultured MCF7 cells as a model, we have developed a novel assay to extract chromatin associated RNAs using magnetophoretic chromatography. The assay could be applied to extract CARs to other cells such as Hela cells [18], non-neoplastic S1 HMT-3522 human mammary epithelial cells [6] as well as human blood white cells [7]. The total assay time including cell separation, chromatin collection and chromatin associated RNA extraction was less than 2h. The SAMNPs could non-specifically adsorb on to the chromatin with the help of isopropanol, to realize the subsequent unbiased extraction of CARs. Magnetophoretic chromatography was used to separate chromatin-SAMNPs complexes from free RNA-SAMNPs complexes and the free SAMNPs. This novel strategy not only improves the chromatin extraction efficiency, but also assists in the extraction of CARs by removing free RNAs. The mild and ultrafast magnetic separation process keeps CARs from degrading. Thus, we have developed an ultrafast, unbiased and cost-effective method for the extraction of CARs, which is essential to ensure the accuracy of transcriptomic analyses.
Highlights.
Salicylic acid coated magnetic nanoparticles has dual functions wherein it can be used to enrich mammalian cells and further capture chromatin after cell lysis.
Magnetophoretic chromatography in a viscous PEG solution removes free RNA from the chromatin.
Compared to RNAs extracted from nucleus, non-coding RNAs can be significantly enriched with the magnetophoretic chromatography method.
An ultra fast and unbiased method for chromatin associated RNA extraction including cell separation, chromatin purification and chromatin associated RNA extraction can be accomplished in less than 2h.
Acknowledgments
This project was partly funded by the W.M. Keck Foundation. USDA-ARS project number 935-42000-049-00D in conjunction with the Center for Food Safety Engineering at Purdue University and the Indiana Clinical and Translational Sciences Institute funded, in part by Grant Number (TR000006) from the NIH are acknowledged.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nakama M, Kawakami K, Kajitani T, Urano T, Murakami Y. DNA–RNA hybrid formation mediates RNAi-directed heterochromatin formation. Genes to Cells. 2012;17:218–233. doi: 10.1111/j.1365-2443.2012.01583.x. [DOI] [PubMed] [Google Scholar]
- 2.Hall LL, Carone DM, Gomez AV, Kolpa HJ, Byron M, Mehta N, Fackelmayer FO, Lawrence JB. Stable C 0 T-1 Repeat RNA is abundant and is associated with euchromatic interphase chromosomes. Cell. 2014;156:907–919. doi: 10.1016/j.cell.2014.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nickerson JA, Krochmalnic G, Wan KM, Penman S. Chromatin architecture and nuclear RNA. Proceedings of the National Academy of Sciences. 1989;86:177–181. doi: 10.1073/pnas.86.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clark MB, Johnston RL, Inostroza-Ponta M, Fox AH, Fortini E, Moscato P, Dinger ME, Mattick JS. Genome-wide analysis of long noncoding RNA stability. Genome research. 2012;22:885–898. doi: 10.1101/gr.131037.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mondal T, Rasmussen M, Pandey GK, Isaksson A, Kanduri C. Characterization of the RNA content of chromatin. Genome research. 2010;20:899–907. doi: 10.1101/gr.103473.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou Z, Irudayaraj J. A native chromatin extraction method based on salicylic acid coated magnetic nanoparticles and characterization of chromatin. Analyst. 2015;140:938–944. doi: 10.1039/c4an01897d. [DOI] [PubMed] [Google Scholar]
- 7.Zhou Z, Cho IIH, Shan Z, Irudayaraj J. Cross-platform detection of epigenetic modifications from extracted chromatin in leucocytes from blood. Analytical Chemistry Research. 2015;4:39–44. [Google Scholar]
- 8.Kwon D, Joo J, Lee J, Park KH, Jeon S. Magnetophoretic Chromatography for the Detection of Pathogenic Bacteria with the Naked Eye. Analytical Chemistry. 2013;85:7594–7598. doi: 10.1021/ac401717f. [DOI] [PubMed] [Google Scholar]
- 9.Kwon D, Lee S, Ahn MM, Kang IS, Park KH, Jeon S. Colorimetric detection of pathogenic bacteria using platinum-coated magnetic nanoparticle clusters and magnetophoretic chromatography. Analytica chimica acta. 2015;883:61–66. doi: 10.1016/j.aca.2015.04.044. [DOI] [PubMed] [Google Scholar]
- 10.Zhou Z, Kadam US, Irudayaraj J. One-stop genomic DNA extraction by salicylic acid-coated magnetic nanoparticles. Anal Biochem. 2013;442:249–252. doi: 10.1016/j.ab.2013.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Y, Hu Z, Qin H, Wei X, Cheng K, Liu F, Wu Ra, Zou H. Highly Efficient Extraction of Cellular Nucleic Acid Associated Proteins in Vitro with Magnetic Oxidized Carbon Nanotubes. Analytical Chemistry. 2012;84:10454–10462. doi: 10.1021/ac302695u. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Y, Hu Z, Qin H, Liu F, Cheng K, Wu Ra, Zou H. Cell Nucleus Targeting for Living Cell Extraction of Nucleic Acid Associated Proteins with Intracellular Nanoprobes of Magnetic Carbon Nanotubes. Analytical Chemistry. 2013;85:7038–7043. doi: 10.1021/ac401269g. [DOI] [PubMed] [Google Scholar]
- 13.Tang L, Zhang W, Su B, Yu B. Long noncoding RNA HOTAIR is associated with motility, invasion, and metastatic potential of metastatic melanoma. BioMed Research International. 2013;2013 doi: 10.1155/2013/251098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun S, Zeng H. Size-Controlled Synthesis of Magnetite Nanoparticles. Journal of the American Chemical Society. 2002;124:8204–8205. doi: 10.1021/ja026501x. [DOI] [PubMed] [Google Scholar]
- 15.Shan Z, Li C, Zhang X, Oakes KD, Servos MR, Wu Q, Chen H, Wang X, Huang Q, Zhou Y, Yang W. Temperature-dependent selective purification of plasmid DNA using magnetic nanoparticles in an RNase-free process. Analytical Biochemistry. 2011;412:117–119. doi: 10.1016/j.ab.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 16.Hontz RD, French SL, Oakes ML, Tongaonkar P, Nomura M, Beyer AL, Smith JS. Transcription of multiple yeast ribosomal DNA genes requires targeting of UAF to the promoter by Uaf30. Molecular and Cellular Biology. 2008;28:6709–6719. doi: 10.1128/MCB.00703-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lv XB, Lian GY, Wang HR, Song E, Yao H, Wang MH. Long Noncoding RNA HOTAIR Is a Prognostic Marker for Esophageal Squamous Cell Carcinoma Progression and Survival. PLoS One. 2013;8:e63516. doi: 10.1371/journal.pone.0063516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou Z, Kadam US, Irudayaraj J. One-stop genomic DNA extraction by salicylic acid-coated magnetic nanoparticles. Analytical Biochemistry. 2013;442:249–252. doi: 10.1016/j.ab.2013.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]


