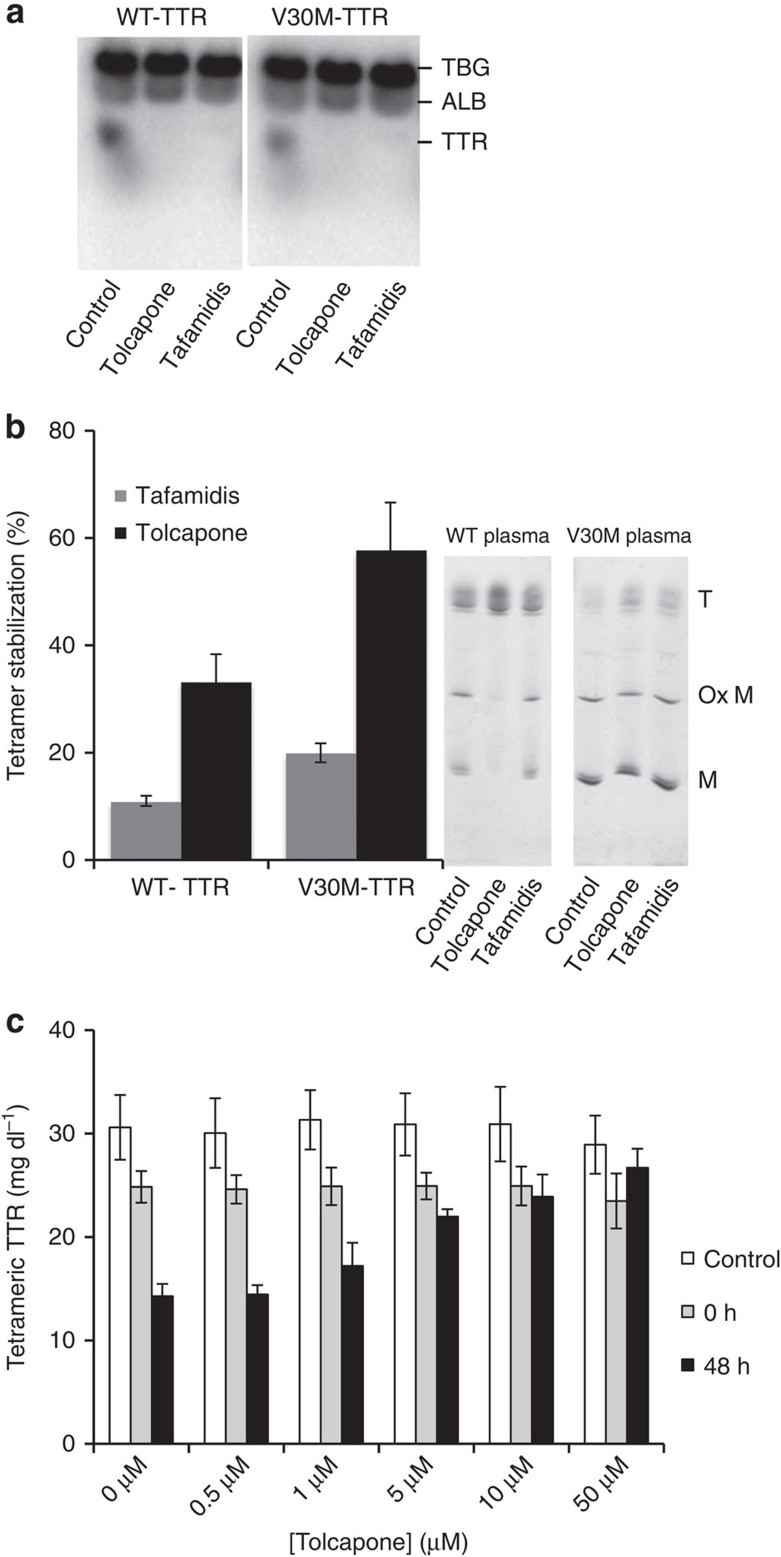
Figure 4. Tolcapone selectively binds and stabilizes WT and V30M-TTR in human plasma.
(a) Human plasma of control or V30M-TTR carriers were incubated with [125I]T4 in the presence or absence of tolcapone or tafamidis (final concentration=1 mM). After 1 h at RT, the plasma proteins were separated by native gel electrophoresis. The gel was subjected to phosphor imaging and the radioactive bands were quantified. The band of TTR bound to radioactive T4 is not present in the plasma samples incubated with the small molecules, indicating an almost complete and selective displacement of T4 from the TTR-binding pocket by the compounds. Uncropped images of the films are shown in Supplementary Fig. 6. (b) Human plasma was incubated with 1.5 mM of tolcapone or tafamidis and tetramer stability assessed by IEF under semi-denaturing conditions62. The increase of the ratio between tetramers and other species in the gels is an indicator of TTR stabilization in drug-treated samples. Quantification of bands shows higher proportion of tetramers in the plasma samples treated with tafamidis and particularly in those treated with tolcapone. ‘T', ‘Ox M' and ‘M' indicate Tetramer, Oxidized Monomers and Monomers, respectively. Error bars indicate s.e.m. (n=3). Uncropped images of the gels are shown in Supplementary Fig. 7. (c) Human plasma samples were incubated with several concentrations of tolcapone for 15 min at RT. After incubation, an aliquot was separated for total TTR determination (control). Urea buffer was added and samples were incubated at RT for 0 or 48 h. After the indicated incubation times, the samples were crosslinked with glutaraldehyde as detailed in the experimental section, and the tetrameric TTR concentration determined by immunoturbidity. Addition of tolcapone to the plasma results in dose-response stabilization of tetrameric TTR. Error bars indicate s.e.m. (n=3).