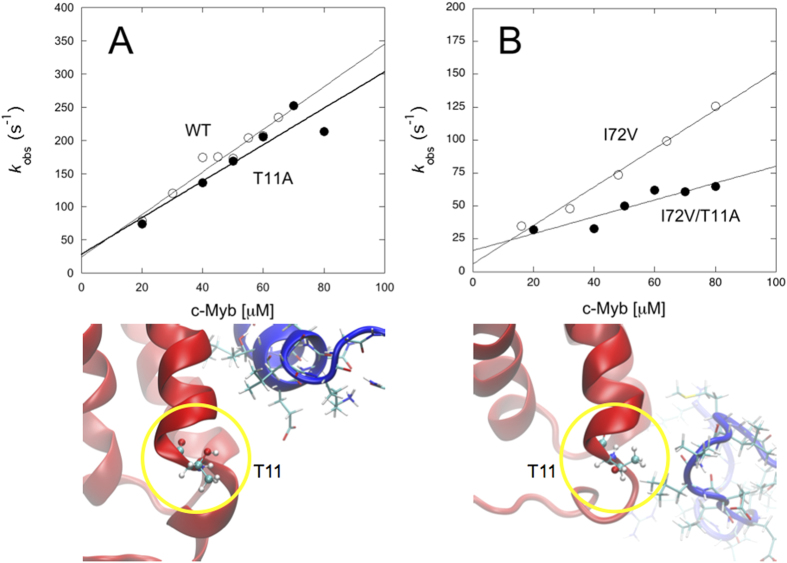
Figure 4. Validation of the transition state ensembles.
To validate the transition state ensembles, we analysed the effects of the T11A substitution on the binding of c-Myb to wild-type KIX (left) and I72V (right). (A) Pseudo-first order binding kinetics of wild type KIX and T11A mutant versus wild type c-Myb. This substitution has a small effect on the binding kinetics, with association and dissociation rate constants equal to kon = 2.7 ± 0.5 μM−1 s−1 and koff = 28 ± 3 s−1 (wild type values kon = 3.2 ± 0.3 μM−1 s−1 and koff = 25 ± 3 s−1). (B) Pseudo-first order binding kinetics of I72V and I72V/T11A versus wild type c-Myb. In this case, substitution in position 11 (highlighted in yellow in Fig. 3) has a detectable effect, the calculated rate constants being kon = 0.6 ± 0.1 μM−1 s−1 and koff = 16 ± 2 s−1 for I72V/T11A, and kon = 1.46 ± 0.09 μM−1 s−1 and koff = 6 ± 1 s−1 for I72V. The structure of the transition states for the KIX wild type and I72V, highlighting the conformation of T11 in the two cases, are shown below each graph.