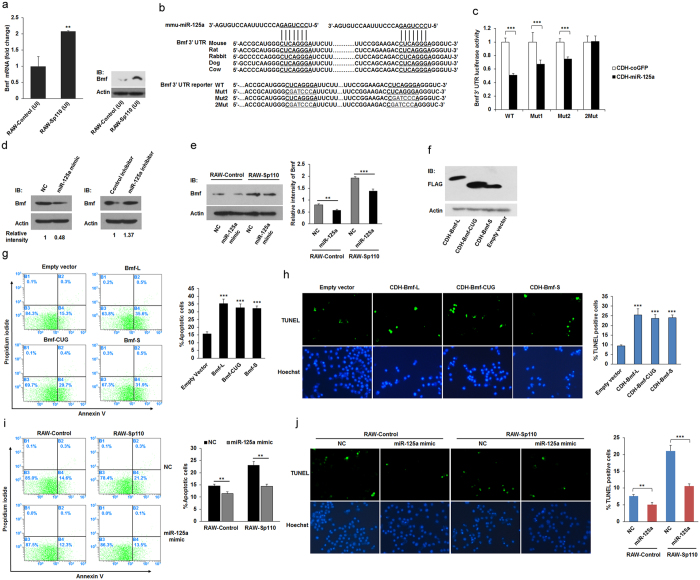
Figure 6. The effect of Sp110-upregulated Bmf on macrophage apoptosis.
(a) The mRNA and protein levels of the Bmf gene in uninfected RAW-Control and RAW-Sp110 cells were determined by qPCR and immunoblots, respectively. (b) Conserved miR-125a binding sites (underlined) in the 3′ UTR of the Bmf mRNA (top), and mutations introduced into the reporter constructs (bottom). (c) Relative luciferase activity in the lysates of HEK293T cells cotransfected with a reporter construct encoding the wild-type (WT) or mutated (Mut) Bmf 3′ UTR and the miRNA expression construct pCDH-miR-125a for 48 h. (d) Endogenous Bmf protein expression in RAW264.7 cells transfected with a mimic or inhibitor of miR-125a-5p for 24 h was determined by immunoblotting. (e) RAW-Control and RAW-Sp110 cells were transfected with the miR-125a mimic for 24 h, after which Bmf expression was examined by immunoblotting. (f) RAW264.7 cells were transduced with lentiviruses encoding different isoforms of Bmf for 30 h, after which Bmf expression was detected by immunoblotting. Apoptosis of RAW264.7 cells transduced with lentiviruses encoding different isoforms of Bmf for 30 h was determined by Annexin-V staining followed by flow cytometric analysis (g) or a TUNEL assay (h). RAW-Control and RAW-Sp110 cells were transfected with the miR-125a mimic, and 6 h after transfection, cells were infected with H37Ra at a multiplicity of infection of 5:1 for 24 h. Cell apoptosis was determined by Annexin-V staining followed by flow cytometric analysis (i) or a TUNEL assay (j). Data represent three independent experiments. *p < 0.05; **p < 0.01; ***p < 0.001. The full-length blots are shown in Supplementary Figure 2.