Fig. 1.
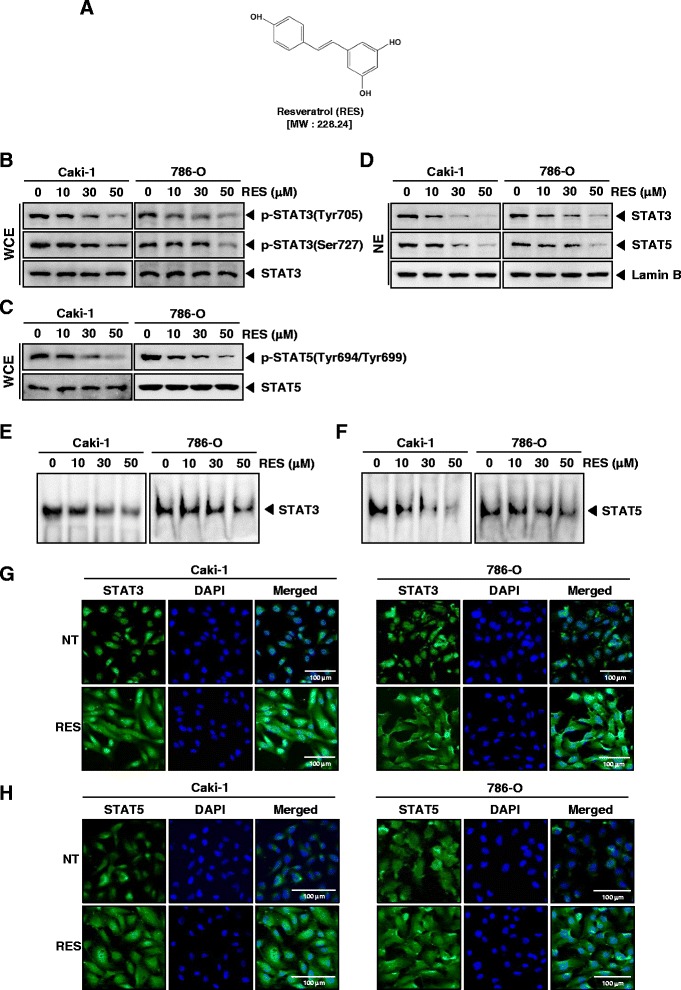
RES suppresses phosphorylation of STAT3 and STAT5 in a dose-dependent manner in RCC cells. a The chemical structure of resveratrol (RES). b and c Caki-1 and 786-O cells (1 × 106 cells/well) were incubated at 37 °C with various indicated concentrations of RES for 6 h. Whole-cell extracts were prepared, then equal amounts of lysates were analyzed by Western blot analysis using antibodies against p-STAT3(Tyr705), p-STAT3(Ser727), STAT3, p-STAT5(Tyr694/Tyr699), and STAT5. The results shown here are representative of three independent experiments. d Caki-1 and 786-O cells (1 × 106 cells/well) were incubated at 37 °C with various indicated concentrations of RES for 6 h. After that, nuclear proteins were extract, equal amounts of lysates were analyzed by Western blot analysis using antibodies against STAT3 and STAT5. The same blots were stripped and reprobed with Lamin B antibody to verify equal protein loading. The results shown here are representative of three independent experiments. e and f RES suppresses STAT3 and STAT5 binding activity in Caki-1 and 786-O cells. After Caki-1 and 786-O cells (1 × 106 cells/well) were incubated at 37 °C with the indicated concentrations of RES for 6 h, analyzed for nuclear STAT3 and STAT5 levels by EMSA. The results shown here are representative of three independent experiments. g and h RES causes inhibition of translocation of STAT3 and STAT5 to the nucleus. Caki-1 and 786-O cells (3 × 104 cells/well) were incubated at 37 °C with 50 μM of RES treatment, the cells were fixed and permeabilized. STAT3 and STAT5 (green) was immunostained with rabbit anti-STAT3 and anti-STAT5 followed by FITC-conjugated secondary antibodies and the nuclei (blue) were stained with DAPI. The third panels show the merged images of the first and second panels. Scale bar = 100 μm. The results shown are representative of two independent experiments
