Abstract
Purpose
Aromatase inhibitors (AIs), adjuvant endocrine therapy for postmenopausal women with hormone receptor positive breast cancer, are associated with bone loss and fractures. Our objectives were to determine if 1) oral bisphosphonate therapy can prevent bone loss in women on an AI and, 2) early changes in bone turnover markers (BTM) can predict later changes in bone mineral density (BMD).
Methods
We conducted a 2 year double-blind, placebo-controlled, randomized trial in 109 postmenopausal women with low bone mass on an aromatase inhibitor (AI-anastrozole, letrozole, or exemestane) for hormone receptor positive breast cancer. Participants were randomized to once weekly risedronate 35 mg or placebo and all received calcium plus vitamin D. The main outcome measures included BMD, BTM [carboxy-terminal collagen crosslinks (CTX) and N-terminal propeptide of type 1 procollagen (P1NP)] and safety.
Results
Eighty-seven percent completed 24 months. BMD increased more in the active treatment group compared to placebo with an adjusted difference at 24 months of 3.9 ± 0.7 percentage points at the spine and 3.2 ± 0.5 percentage points at the hip (both p<0.05). The adjusted difference between the active treatment and placebo groups were 0.09 ± 0.04 nmol/LBCE for CTX and 23.3 ± 4.8 µg/mL for P1NP (both p<0.05). Women with greater 12-month decreases in CTX and P1NP in the active treatment group had a greater 24-month increase in spinal BMD (p<0.05). The oral therapy was safe and well tolerated.
Conclusion
In postmenopausal women with low bone mass and breast cancer on an AI, the oral bisphosphonate risedronate maintained skeletal health.
Keywords: Clinical trials, antiresorptives, DXA, Biochemical markers of bone turnover
Introduction
Aromatase inhibitors (AIs) have become standard adjuvant endocrine therapy for postmenopausal women with hormone receptor positive breast cancer.(1–3) AIs inhibit peripheral conversion of androgens to estrogens resulting in lower levels of estrogen. Because estrogen inhibits bone resorption, AIs contribute to bone loss and osteoporosis.(1–3)
We have previously demonstrated that the oral bisphosphonate risedronate can prevent bone loss in newly postmenopausal women with breast cancer.(4) In that study women were included with or without tamoxifen, an antiestrogen or on AI concomitant therapy. Due to a switch in the standard of care during the 24 months of the trial, a portion of women were switched from tamoxifen to an AI or started on an AI by their private physician. Despite this challenge, we found that women with breast cancer with or without AI therapy received skeletal benefit from an oral bisphosphonate. Furthermore, women on a bisphosphonate and not on an AI obtained the greatest improvement in bone density.
There have been several pivotal studies that demonstrate that intravenous (IV) bisphosphonates improve bone density with women with breast cancer on a specific aromatase inhibitor (AI). The Z-FAST and ZO-FAST trials included postmenopausal women with early breast cancer receiving adjuvant letrozole and found significant increases in spine bone mineral density (BMD) between upfront or delayed zoledronic acid.(5, 6) The ABCSG-12 study included premenopausal women with hormone receptor positive early breast cancer on tamoxifen or anastrozole (both with goserelin) and reported favorable spine BMD responses in those randomized to IV zoledronic acid compared to placebo.(7)
Studies that have included oral bisphosphonates have also demonstrated favorable outcomes in maintaining bone mass in women on a specified AI. (8–11) The Sabre study examined postmenopausal women with hormone receptor positive breast cancer on the AI anastrozole, and were classified as low, moderate or high risk for an osteoporotic fracture as assessed by BMD T-scores and clinical risk factors.(8) The moderate fracture risk group of women on anastrozole, with T-scores from −1.0 to −2.0, was randomized to risedronate or placebo for 2 years. They reported that women receiving adjuvant anastrozole for breast cancer at moderate risk for fracture had favorable effects in BMD with risedronate compared with placebo. Similar favorable BMD outcomes were reported in the IBIS-II trial.(12) This study followed postmenopausal women with osteoporosis on anastrozole for 3 years but 36% dropped out and patients with significant bone loss were excluded from the per protocol analysis. Subsequently the ARBI trial, centered in Athens, Greece found that once weekly risedronate did not improve BMD at 12 months, but did at 24 months.(11)
Little data are available on postmenopausal women with hormone receptor positive breast cancer and low bone mass on other AIs besides anastrozole.(8, 9, 11–14) Because women often have side effects from the initial AI and are switched to an alternative AI,(15, 16) it is important to determine the impact of bone loss on available AI preparations. The Risedronate Effect on Bone in women with Breast CAncer 2 (REBBeCA2) was designed to examine the preservation of bone mass with an oral bisphosphonate, in women with osteopenia or low bone mass on a non-specified AI for adjuvant therapy and allowed participants to switch AI preparations during the trial to mimic conventional clinical care thus providing greater generalizability. Furthermore, our second goal was to determine if early changes in biochemical markers of bone turnover were predictive of longer term changes in BMD in this cohort.
Study Design
The study was a 24 month double-blind, placebo-controlled, randomized clinical trial. Patients were randomly assigned to receive oral risedronate 35 mg once weekly or placebo. Compliance was assessed by pill count. Dietary calcium intake was assessed with a validated questionnaire (17) and all women received daily calcium up to 1200 mg daily by diet and/or supplement.(18, 19) The calcium supplement contained calcium carbonate 500 mg plus vitamin D 200 IU. Participants from the greater Pittsburgh, Pennsylvania area were enrolled and treated between January 2008 and March 2013 (ClinicalTrials.gov Identifier: NCT00485953).
Materials and Methods
Participants
We enrolled postmenopausal women with hormone receptor positive breast cancer over age 55 years, currently receiving an AI including anastrozole, letrozole, or exemestane. Participants were permitted to switch their AI. Postmenopausal status was determined by history. Participants were included if they had low bone mass as classified by the World Health Organization (T-score between −1.0 and −2.5 at the spine or hip(20)), were not treated with a bisphosphonate in the previous year, and had no illnesses or were on no other medications known to affect bone and mineral metabolism such as glucocorticoids or certain antiseizure medications. If the patient had an initial BMD T-score in the osteoporotic range (T-score ≤ −2.5) or an adult fragility fracture they were counseled about options for therapy versus participation in the trial. They were allowed to participate in the trial after discussion and approval from their health care professional and/or oncologist. The protocol was approved by the University of Pittsburgh Institutional Review Board and all participants provided written informed consent before participation.
Randomization and Blinding
The study biostatistician randomized participants in a 1:1 ratio using random block sizes of 2 and 4. The independent research pharmacist provided identically-appearing active drug or placebo. Investigators, study personnel, providers, and participants were blind to treatment assignment.
Clinical Protocol
Study visits occurred at baseline, 6, 12, 18, and 24 months.
Outcome Variables
The primary outcome variables were the changes in spine and hip BMD at 24 months. Additional outcomes included BMD changes at 12 months and changes in dual-energy x-ray absorptiometry (DXA) was performed using a Discovery densitometer (Hologic Inc., Bedford, MA). Our precision ranged from 1.2 to1.9% at these skeletal sites.(21) Measured skeletal sites included the hip (total hip, femoral neck), spine (posterior-anterior), and total body. The markers of bone turnover markers (BTMs) included a marker for bone resorption assessed by serum C-telopeptide crosslinks type I collagen (CTX, Crosslaps, Osteometer Biotech, Herlev, Denmark). Bone formation was assessed by serum intact N-terminal propeptide type I procollagen (P1NP, Orion Diagnostica, Espoo, Finland). Serum 25-hydroxy vitamin D was assessed by liquid chromatography/mass spectrometry.
Sample Size
Standard deviations of BMD improvement is approximately 4%.(22–24) We conservatively assumed a one-year dropout rate of 20%, and zero BMD change in dropouts. Under this assumption, we conservatively sought to detect 75% of effect actually attained by others(25) or 2.76%. With 55 participants per arm, we estimated to have 89% power to detect statistical significance of such an effect in a two-tailed test at α=0.05.
Statistical Analysis
Analyses were performed on an intention-to-treat basis. To compare baseline characteristics between subjects in the active and placebo arms, we used independent sample t-, chi square and Fisher’s exact tests. For the main analysis, we fitted a series of linear mixed models, using raw and percent change between baseline and follow-up assessment in each of the BMD and biomarker measures as the dependent variable; treatment arm (active/placebo), follow-up assessment (6,12,18,24 months) and their interaction as fixed effects of interest; baseline value of the measure as a fixed effect covariate; and a subject random effect to account for multiple measurements from the same participant over time and the resulting non-independence of observations. We used appropriately constructed contrasts to compare treatment arms at each of the 6-, 12-, 18-, and 24-month assessments. We assessed sensitivity of the results to both last-value-carried-forward and multiple imputation(26) approaches to missing data. To examine association between early changes in markers of bone turnover with more distal changes in BMD, we computed correlation coefficients between 12-month change in markers and 24-month changes in BMD; and additionally compared 24-month BMD changes across tertiles of 12-month marker changes using analysis of variance. SAS® version 9.3 (SAS Institute, Cary, North Carolina) was used for all statistical analyses.
Results
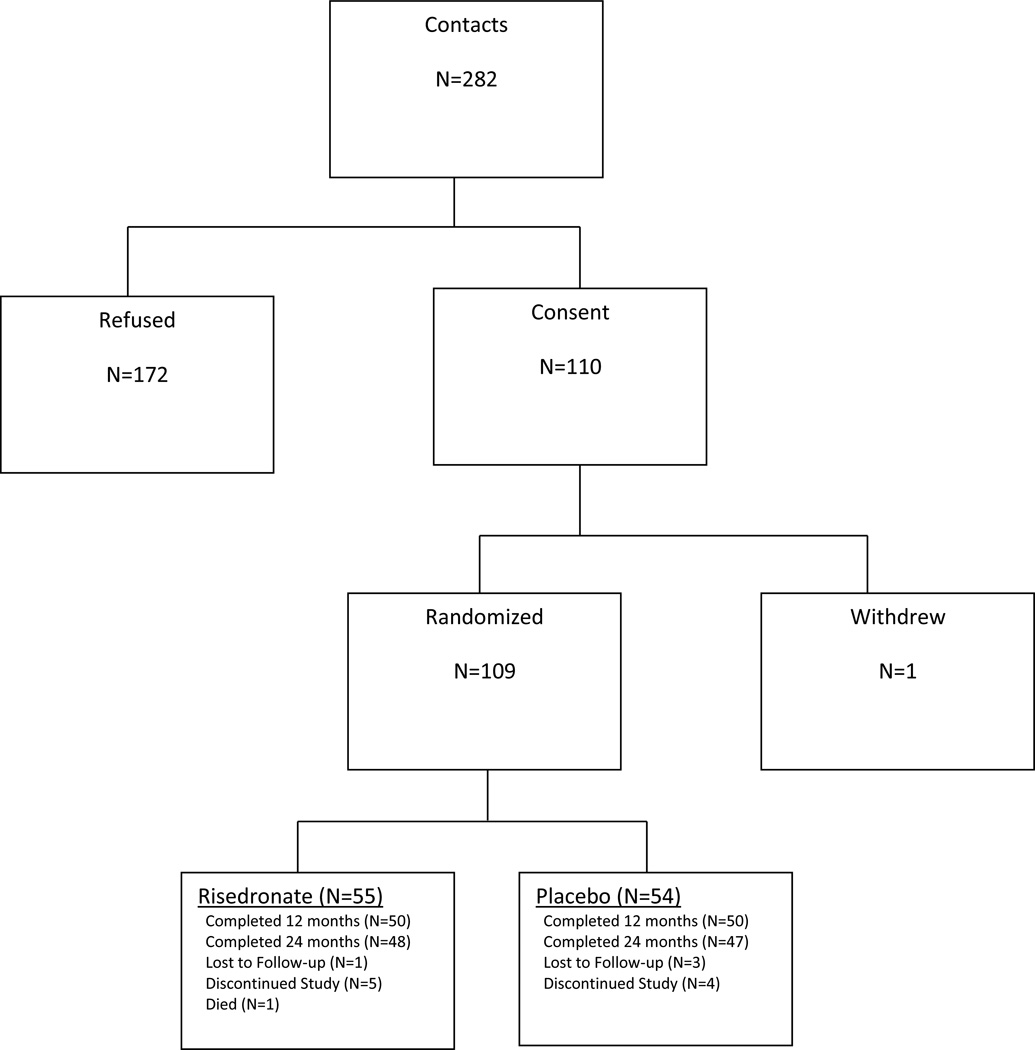
During the recruitment period 280 women were screened via a phone interview, 110 were evaluated for inclusion by the study staff with coordination from their primary care physician and/or oncologist, and 109 were randomized (Figure 1). At randomization, 77% were currently taking anastrozole, 15% letrozole, and 8% exemestane. Twelve (11%) women reported use of more than one AI prior to randomization and 9 (8%) reported changing AI preparations at some point during the study. At baseline the mean age was 70.5 years and there were no baseline differences between the groups in age, BMI, calcium/vitamin D intake or BMD (Table 1). Eighty-seven percent completed 24 months of the study. Compliance by pill count was assessed every 6 months and considered compliant if women took at least 80% of the pills in the previous 6-month period. During the four 6-month periods of the 24 month study, compliance ranged from 90–98% in the risedronate group and 89–96% in the placebo group.
Figure 1.
Enrollment and Study Design Flow Chart- CONSORT Diagram
Table 1.
Baseline Characteristics, Bone Mineral Density and Bone Turnover
| Variable | Risedronate (N=55) | Placebo (N=54) | P-value |
|---|---|---|---|
| Age (years) | 65 ± 1 | 64 ± 1 | 0.58 |
| Menopausal age (years) | 50 ± 1 | 51 ± 1 | 0.65 |
| AI use prior to study (days) | 747 ± 101 | 684 ± 87 | 0.64 |
| Body mass index (kg/m2) | 31 ± 3 | 31 ± 3 | 0.905 |
| Daily calcium (mg/d) | 743 ± 48 | 827 ± 54 | 0.25 |
| Breast cancer treatment | |||
| Lumpectomy, N (%) | 43 (81) | 43(79) | 0.84 |
| Mastectomy, N (%) | 19 (35) | 17(32) | 0.73 |
| Axillary node removal | 39(75) | 48(89) | 0.06 |
| Radiation therapy, N (%) | 42 (76) | 49 (91) | 0.09 |
| Chemotherapy, N (%) | 17 (32) | 27 (50) | 0.077 |
| Aromatase inhibitor | |||
| Anastrozole, N (%) | 45 (82) | 39 (72) | 0.261 |
| Letrozole, N (%) | 4 (7) | 12 (22) | 0.033 |
| Exemestane, N (%) | 6 (11) | 3 (6) | 0.489 |
| Bone metabolism | |||
| Calcium (mg/dl) | 9.8 ± 0.1 | 9.7 ± 0.1 | 0.22 |
| 25 hydroxyvitamin D (ng/mL) | 36 ± 2 | 36 ± 2 | 1.00 |
| Bone Mineral Density (g/cm2) | |||
| Spine | 0.980 ± 0.019 | 0.994 ± 0.017 | 0.60 |
| Total hip | 0.866 ± 0.014 | 0.874 ± 0.014 | 0.69 |
| Femoral neck | 0.744 ± 0.013 | 0.744 ± 0.013 | 1.00 |
| T-score (SD) | |||
| PA Spine | −0.64 ± 0.16 | −0.45 ± 0.15 | 0.39 |
| Total hip | −0.67 ± 0.12 | −0.58 ± 0.11 | 0.60 |
| Femoral neck | −1.01 ± 0.11 | −0.95 ± 0.16 | 0.75 |
| Bone turnover markers | |||
| CTX (nmol/LBCE) | 0.38 ± 0.02 | 0.46 ± 0.03 | 0.04 |
| P1NP (µg/mL) | 62.05 ± 3.04 | 62.61 ± 3.51 | 0.9055 |
Bone Mineral Density
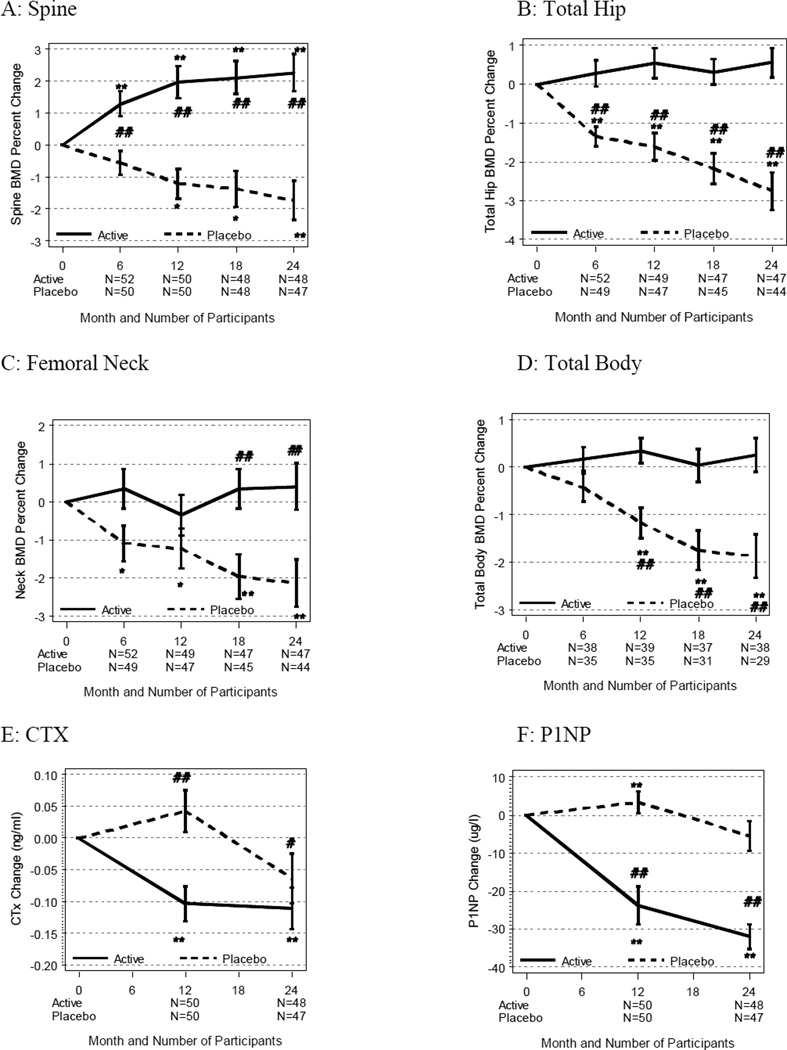
Spine BMD increased in the active treatment group decreased in the placebo over 12 months (2.0±0.5 vs. −1.2±0.5%, mean ± SE, p<0.0001) and over 24 months (2.3±0.6 vs. −1.7±0.6, p<0.0001); the adjusted difference was 3.9±0.7 percentage points in favor of the active group at 24 months (p<0.0001, Figure 2). Total hip BMD increased more in the active treatment group than placebo, both at 12 months (0.5±0.4 vs. −1.6±0.4; p<0.0001) and 24 months (0.6±0.4 vs. −2.7±0.5, p<0.0001); the adjusted difference at 24 months was 3.2±0.5 percentage points, p < 0.0001.
Figure 2.
Mean ± SE percent change in bone mineral density in spine (A), total hip (B), femoral neck (C), total body (D) and the absolute change in biochemical markers of bone turnover including CTX [(E), nmol/L BCE] and P1NP [(F), µg/mL] from baseline to 24 months (unadjusted). * p<0.05, ** p<0.01 change from baseline using paired t-test. #p<0.05, ##p<0.01 for comparison between risedronate (solid line) and placebo (dashed line) groups using linear mixed models.
Similar differences were observed in the femoral neck with an adjusted difference of 2.6±0.8 percentage points at 24 months (p=0.0009). We also observed significant differences in total body (2.4 percentage point adjusted difference at 24 months, p<0.0001). Results were descriptively similar among those on different AIs.
Biochemical Markers of Bone Turnover
As assessed by the marker of bone resorption, CTX decreased in the active treatment group at 12 and 24 months (p<0.01, Figure 2) and did not significantly change in the placebo group, with an adjusted difference of 0.09±0.04 nmol/LBCE at 24 months (p=0.0157). As expected, the marker of bone formation, P1NP, decreased in the active treatment group at 12 and 24 months (p<0.0001), with an adjusted difference of 27.1±4.7 µg/mL at 12 months (p<0.0001) and 23.3±4.8 at 24 months (p<0.0001, Figure 2). There were no significant correlations between the baseline BTMs and subsequent changes in BMD.
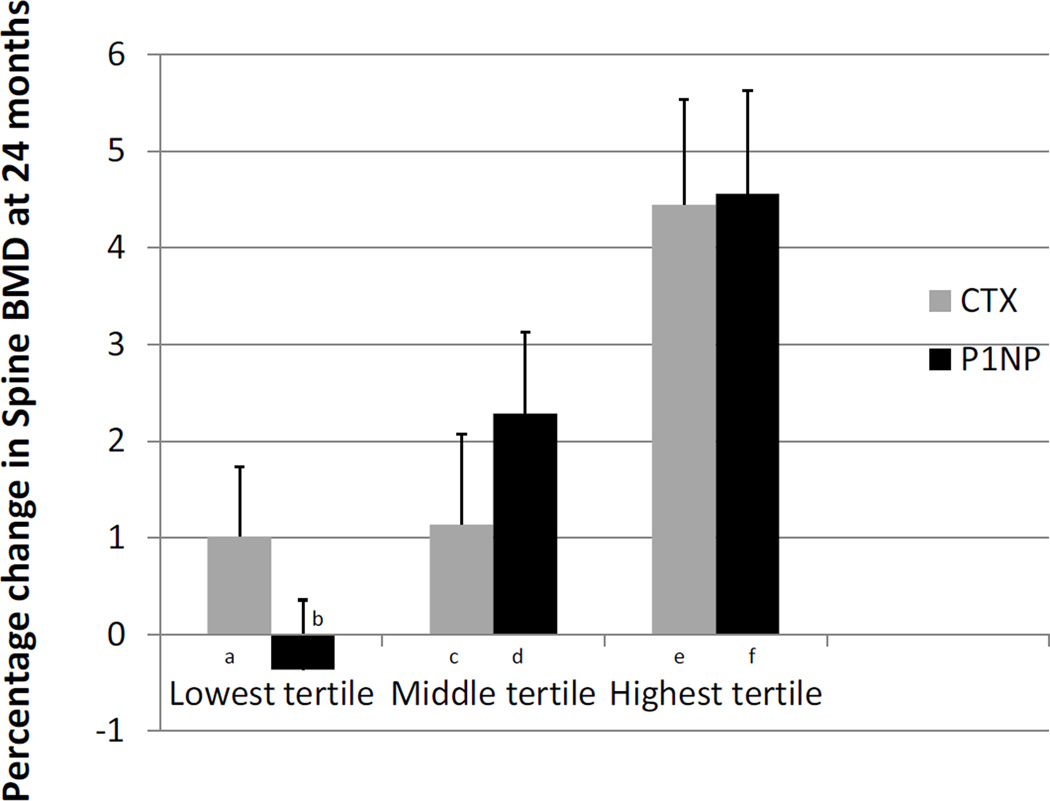
In the risedronate group, the 12-month changes in markers were associated with the 1- and 2-year percent changes in BMD (Table 2). Women in the highest (≥43%) 12-month CTX decrease tertile had a 3.3 to 3.4 percentage point greater 24-month spine BMD improvement compared to those in other tertiles (Figure 3; p<0.05). Similarly women in the highest two (37–50% and ≥50%) 12-month P1NP decrease tertiles had a 2.7 to 4.9 percentage point greater 24-month spine BMD improvement compared to those in the lowest tertile (p<0.05). Similar trends were observed for P1NP change and total hip BMD change (data not shown).
Table 2.
Correlations Between 12-month Change in Markers of Bone Turnover and 12–24-Month Percent Changes in Bone Mineral Density Among the Women Receiving Active Treatment
| 12-Month CTX Change | 12-Month P1NP Change | |||
|---|---|---|---|---|
| BMD Site | 12-Month BMD Percent Change |
24-Month BMD Percent Change |
12-Month BMD Percent Change |
24-Month BMD Percent Change |
| PA Spine | −0.38** | −0.32* | −0.30* | −0.43** |
| Total Hip | −0.22 | −0.17 | −0.37** | −0.20 |
| Femoral Neck | −0.04 | −0.26 | 0.09 | −0.12 |
| Total Body | −0.37* | −0.42** | −0.20 | −0.31 |
P<0.05,
p<0.01
Figure 3.
Percent change in spine bone mineral density (BMD) after 24 months of risedronate grouped by percentage decrease in bone turnover markers (in tertiles) at 12 months. Results as mean ± SEM. For CTX (overall ANOVA pairwise comparison p<0.05), group a) lowest tertile (<13%), b) middle tertile (13 – <43%) and c) highest tertile (>43% decrease). For P1NP (overall ANOVA pairwise comparison p<0.01), group d) lowest tertile (<37%), e) middle tertile (37–50%), and f) highest tertile (>50% decrease). Significance between tertiles: a vs. c (p<0.01), b vs. c (p<0.05), d vs. e (p<0.05), d vs. f (p<0.01), and e vs. f (p=0.07).
Adverse Events
Twenty-four percent of participants had a serious adverse event and 94% had a nonserious adverse event. There were no differences between the groups (Table 3).
Table 3.
Adverse Events
| Adverse Event | Risedronate N (%) |
Placebo N (%) |
P-value |
|---|---|---|---|
| Breast related | 5 (9) | 4 (7) | 1.00 |
| Gastrointestinal | 4 (7) | 13 (24) | 0.019 |
| Cardiovascular | 8 (14) | 11 (20) | 0.459 |
| Respiratory | 4(7) | 10(18) | 0.093 |
| Musculoskeletal | 27 (49) | 33 (61) | 0.250 |
| Serious | 10 (18) | 16 (30) | 0.183 |
| Nonserious | 52(95) | 50(93) | 0.716 |
Discussion
We found that once weekly oral risedronate improved bone density and decreased bone turnover in postmenopausal women with hormone receptor positive breast cancer on an AI over 2 years. Furthermore, the early decrease in BTMs predicted the increase in vertebral BMD. In comparison to previous studies that used IV bisphosphonates and only allowed one AI, this study allowed use of 3 different AIs during the study and used an oral once weekly bisphosphonate.
Studies in postmenopausal women on AIs for breast cancer with lower bone mineral density or osteoporosis using IV bisphosphonates have also demonstrated improvement in BMD with antiresorptive medication. Two large trials using IV zoledronic acid every 4 months in women on letrozole adjuvant therapy compared immediate to delayed therapy and reported skeletal benefits from therapy(7, 27) but neither trial was designed to demonstrate fracture reduction. A meta-analysis with 15 trials demonstrated a 22% fracture reduction with IV zoledronic acid therapy (28) and other reviews support use of bisphosphonates in women with breast cancer on an AI.(29, 30) Parental therapy with denosumab subcutaneously every 6 months for 2 year also found improvements in BMD with therapy, but no difference in fractures.(31, 32) These studies with a parental antiresorptive therapy and previous trials with oral risedronate, have only allowed a single AI preparation. This is the first trial to allow the spectrum of available AIs and to allow women to change preparations which is the standard of care and provides greater generalizability. Roughly 75% were on anastozole, and 25% were on the other available AIs. Furthermore, roughly 11% had changed AIs before the trial and 8% switched during the study.
Previous studies on the prevention of bone loss with antiresorptive therapy in women with breast cancer on an AI have not explored the relationship between early decreases in BTMs and longer term changes in bone density. We found that the decreases in BTMs at 1 year predict the improvements in BMD at 2 years. We have previously demonstrated this in postmenopausal women on alendronate therapy.(33, 34) Assessment of a blood test halfway through the course of therapy may be clinically useful to reassure patients and physicians that the BMD trajectory is in the right direction, but larger studies are needed to validate this.
Our study had several limitations
The duration of the study was only 2 years. We only included women with low bone mass who were unlikely to fracture and we were not powered for fracture efficacy. However our study had several strengths. We performed the study at a single center on a single machine, thereby reducing extraneous variability due to site and equipment. Our compliance (defined as using ≥80% of the pills) was at least 90% during each of the 6-month periods between assessments. Eighty-seven percent completed the 2 year study. Furthermore, we had performed analyses based on intention-to-treat; results were similar when multiple approaches for accounting for missing data were employed. Finally the reduction in biochemical markers of bone turnover, provide an extra level of support for the validity of increases we observed in BMD.
In summary, we found that an oral once weekly bisphosphonate, risedronate improved bone density and decreased bone turnover in postmenopausal with hormone receptor positive breast cancer on an AI preparation. The bisphosphonate was well tolerated and provides a therapeutic option to maintain skeletal integrity in this cohort.
Acknowledgments
The study was funded by grant support form Procter and Gamble and the Alliance for Better Bone Health and Warner Chilcott who supplied the drug and matching placebo. Support was also provided by NIH grants K24DK062895, T32AG021885 and P30AG024827. All authors had access to this manuscript and they fulfilled the following respective roles: SG-PI, manuscript preparation, data analysis, manuscript editing; KV-patient visits, recruitment, data collection; SKP- data analysis; AB- patient recruitment; BL- patient recruitment; J vL- patient visits, recruitment; RJ-patient recruitment; SLP-patient recruitment; PR- patient recruitment. Susan L. Greenspan is the corresponding author and accepts responsibility for the integrity of the data analysis.
Grant Funding: Susan L. Greenspan has the following grant funding- NIH, PCORI, Adam Brufsky has the following grant funding - NIH/NCI; G. J. van Londen has the following grant funding -NIH; Rachel C. Jankowitz has the following grant funding - NSABP Foundation, Komen Foundation; Subashan Perera has the following grant funding -NIH, PCORI
Footnotes
Disclosure Statement: Susan L. Greenspan none present a conflict of interest; Eli Lilly, Amgen; Karen T. Vujevich, Barry C. Lembersky, Shannon L. Puhalla, and Priya Rastogi have no conflict of interest to declare; Adam Brufsky none present a conflict of interest; G. J. van Londen none present a conflict of interest; Rachel C. Jankowitz none present a conflict of interest; Subashan Perera none present a conflict of interest
Reference List
- 1.Becker T, Lipscombe L, Narod S, Simmons C, Anderson GM, Rochon PA. Systematic review of bone health in older women treated with aromatase inhibitors for early-stage breast cancer. J Am Geriatr Soc. 2012;60(9):1761–1767. doi: 10.1111/j.1532-5415.2012.04107.x. Epub 2012/09/19. PubMed PMID: 22985145. [DOI] [PubMed] [Google Scholar]
- 2.Bauer M, Bryce J, Hadji P. Aromatase inhibitor-associated bone loss and its management with bisphosphonates in patients with breast cancer. Breast cancer (Dove Medical Press) 2012;4:91–101. doi: 10.2147/BCTT.S29432. Epub 2012/01/01. PubMed PMID: 24367197; PubMed Central PMCID: PMCPmc3846762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hadji P. Guidelines for Osteoprotection in Breast Cancer Patients on an Aromatase Inhibitor. Breast care (Basel, Switzerland) 2010;5(5):290–296. doi: 10.1159/000321426. Epub 2010/01/01. PubMed PMID: 21779210; PubMed Central PMCID: PMCPmc3132952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greenspan SL, Brufsky A, Lembersky BC, Bhattacharya R, Vujevich KT, Perera S, et al. Risedronate Prevents Bone Loss in Breast Cancer Survivors: a 2 Year, Randomized, Double-Blind, Placebo-Controlled Clinical Trial. J Clin Oncol. 2008;26(16):2644–2652. doi: 10.1200/JCO.2007.15.2967. PubMed PMID: 18427147; PubMed Central PMCID: PMCPmc3992822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brufsky AM, Harker WG, Beck JT, Bosserman L, Vogel C, Seidler C, et al. Final 5-year results of Z-FAST trial: adjuvant zoledronic acid maintains bone mass in postmenopausal breast cancer patients receiving letrozole. Cancer. 2012;118(5):1192–1201. doi: 10.1002/cncr.26313. Epub 2011/10/12. PubMed PMID: 21987386. [DOI] [PubMed] [Google Scholar]
- 6.Llombart A, Frassoldati A, Paija O, Sleeboom HP, Jerusalem G, Mebis J, et al. Immediate Administration of Zoledronic Acid Reduces Aromatase Inhibitor-Associated Bone Loss in Postmenopausal Women With Early Breast Cancer: 12-month analysis of the E-ZO-FAST trial. Clin Breast Cancer. 2012;12(1):40–48. doi: 10.1016/j.clbc.2011.08.002. Epub 2011/10/22. PubMed PMID: 22014381. [DOI] [PubMed] [Google Scholar]
- 7.Gnant M, Mlineritsch B, Luschin-Ebengreuth G, Kainberger F, Kassmann H, Piswanger-Solkner JC, et al. Adjuvant endocrine therapy plus zoledronic acid in premenopausal women with early-stage breast cancer: 5-year follow-up of the ABCSG-12 bone-mineral density substudy. Lancet Oncol. 2008;9(9):840–849. doi: 10.1016/S1470-2045(08)70204-3. Epub 2008/08/23. PubMed PMID: 18718815. [DOI] [PubMed] [Google Scholar]
- 8.Van Poznak C, Hannon RA, Mackey JR, Campone M, Apffelstaedt JP, Clack G, et al. Prevention of aromatase inhibitor-induced bone loss using risedronate: the SABRE trial. J Clin Oncol. 2010;28(6):967–975. doi: 10.1200/JCO.2009.24.5902. Epub 2010/01/13. PubMed PMID: 20065185. [DOI] [PubMed] [Google Scholar]
- 9.Markopoulos C, Tzoracoleftherakis E, Koukouras D, Venizelos B, Zobolas V, Misitzis J, et al. Age effect on bone mineral density changes in breast cancer patients receiving anastrozole: results from the ARBI prospective clinical trial. J Cancer Res Clin Oncol. 2012;138(9):1569–1577. doi: 10.1007/s00432-012-1233-z. Epub 2012/05/04. PubMed PMID: 22552718; PubMed Central PMCID: PMCPmc3418493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Poznak C. Managing bone mineral density with oral bisphosphonate therapy in women with breast cancer receiving adjuvant aromatase inhibition. Breast Cancer Res. 2010;12(3):110. doi: 10.1186/bcr2584. Epub 2010/06/30. PubMed PMID: 20584345; PubMed Central PMCID: PMCPmc2917025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Markopoulos C, Tzoracoleftherakis E, Polychronis A, Venizelos B, Dafni U, Xepapadakis G, et al. Management of anastrozole-induced bone loss in breast cancer patients with oral risedronate: results from the ARBI prospective clinical trial. Breast Cancer Res. 2010;12(2):R24. doi: 10.1186/bcr2565. Epub 2010/04/20. PubMed PMID: 20398352; PubMed Central PMCID: PMCPmc2879572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sestak I, Singh S, Cuzick J, Blake GM, Patel R, Gossiel F, et al. Changes in bone mineral density at 3 years in postmenopausal women receiving anastrozole and risedronate in the IBIS-II bone substudy: an international, double-blind, randomised, placebo-controlled trial. Lancet Oncol. 2014;15(13):1460–1468. doi: 10.1016/S1470-2045(14)71035-6. Epub 2014/12/03. PubMed PMID: 25456365. [DOI] [PubMed] [Google Scholar]
- 13.Sergi G, Pintore G, Falci C, Veronese N, Berton L, Perissinotto E, et al. Preventive effect of risedronate on bone loss and frailty fractures in elderly women treated with anastrozole for early breast cancer. J Bone Miner Metab. 2012;30(4):461–467. doi: 10.1007/s00774-011-0341-1. Epub 2011/12/14. PubMed PMID: 22160398. [DOI] [PubMed] [Google Scholar]
- 14.Lester JE, Dodwell D, Purohit OP, Gutcher SA, Ellis SP, Thorpe R, et al. Prevention of anastrozole-induced bone loss with monthly oral ibandronate during adjuvant aromatase inhibitor therapy for breast cancer. Clin Cancer Res. 2008;14(19):6336–6342. doi: 10.1158/1078-0432.CCR-07-5101. Epub 2008/10/03. PubMed PMID: 18829518. [DOI] [PubMed] [Google Scholar]
- 15.Henry NL, Azzouz F, Desta Z, Li L, Nguyen AT, Lemler S, et al. Predictors of aromatase inhibitor discontinuation as a result of treatment-emergent symptoms in early-stage breast cancer. J Clin Oncol. 2012;30(9):936–942. doi: 10.1200/JCO.2011.38.0261. Epub 2012/02/15. PubMed PMID: 22331951; PubMed Central PMCID: PMCPmc3341106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Briot K, Tubiana-Hulin M, Bastit L, Kloos I, Roux C. Effect of a switch of aromatase inhibitors on musculoskeletal symptoms in postmenopausal women with hormone-receptor-positive breast cancer: the ATOLL (articular tolerance of letrozole) study. Breast Cancer Res Treat. 2010;120(1):127–134. doi: 10.1007/s10549-009-0692-7. Epub 2009/12/26. PubMed PMID: 20035381. [DOI] [PubMed] [Google Scholar]
- 17.Dawson-Hughes B, Jacques P, Shipp C. Dietary calcium intake and bone loss from the spine in healthy postmenopausal women. Am J Clin Nutr. 1987;46:685–687. doi: 10.1093/ajcn/46.4.685. [DOI] [PubMed] [Google Scholar]
- 18.DRI Dietary reference intakes for calcium and vitamin D. Washington, D.C.: National Academies Press; 2011. Committee to Review Dietary Reference Intakes for Vitamin D, Calcium. Food, Nutrition B. [Google Scholar]
- 19.Cosman F, de Beur SJ, LeBoff MS, Lewiecki EM, Tanner B, Randall S, et al. Clinician's Guide to Prevention and Treatment of Osteoporosis. Osteoporos Int. 2014;25(10):2359–2381. doi: 10.1007/s00198-014-2794-2. Epub 2014/09/04. PubMed PMID: 25182228; PubMed Central PMCID: PMCPmc4176573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kanis JA. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: synopsis of a WHO report. WHO Study Group. Osteoporos Int. 1994;4:368–381. doi: 10.1007/BF01622200. [DOI] [PubMed] [Google Scholar]
- 21.Varney LF, Parker RA, Vincelette A, Greenspan SL. Classification of osteoporosis and osteopenia in postmenopausal women is dependent on site-specific analysis. J Clin Densitom. 1999;2:275–283. doi: 10.1385/jcd:2:3:275. [DOI] [PubMed] [Google Scholar]
- 22.Coleman RE, Banks LM, Girgis SI, Vrdoljak E, Fox J, Porter LS, et al. Skeletal effect of exemestane in the Intergroup Exemestane Study (IES) 2 year bone mineral density (BMD) and bone biomarker data; Presented at 28th Annual San Antonio Breast Cancer Symposium; 2005. [Google Scholar]
- 23.Howell A. Effect of anastrozole on bone mineral density: 2-year results of the 'Arimidex' (anastrozole), Tamoxifen, Alone or in Combination (ATAC) trial; Presented at the San Antonio Breast Cancer Symposium 2003; 2003. [Google Scholar]
- 24.Lonning PE, Geisler J, Krag LE, Erikstein B, Bremnes Y, Hagen AI, et al. Effects of Exemestane Administered for 2 Years Versus Placebo on Bone Mineral Density, Bone Biomarkers, and Plama Lipids in Patients With Surgically Resected Early Breast Cancer. J Clin Oncol. 2005;23:5126–5137. doi: 10.1200/JCO.2005.07.097. [DOI] [PubMed] [Google Scholar]
- 25.Brufsky A, Harker W, Beck J, Carroll R, Tan-Chiu E, Seidler C, et al. Zoledronic acid (ZA) effectively inhibits cancer treatment-induced bone loss (CTIBL) in postmenopausal women (PMW) with early breast cancer (BCa) receiving adjuvant Letrozole (Let): 12 mos BMD results of the Z-FAST trial; Presented at the annual meeting of the American Society of Clinical Oncology (ASCO); 2005. [Google Scholar]
- 26.Rubin DB. Multiple imputation for nonresponse in surveys. New York, NY: John Wiley & Sons, Ltd.; 1987. [Google Scholar]
- 27.Brufsky AM, Bosserman LD, Caradonna RR, Haley BB, Jones CM, Moore HC, et al. Zoledronic acid effectively prevents aromatase inhibitor-associated bone loss in postmenopausal women with early breast cancer receiving adjuvant letrozole: Z-FAST study 36-month follow-up results. Clin Breast Cancer. 2009;9(2):77–85. doi: 10.3816/CBC.2009.n.015. Epub 2009/05/13. PubMed PMID: 19433387. [DOI] [PubMed] [Google Scholar]
- 28.Valachis A, Polyzos NP, Coleman RE, Gnant M, Eidtmann H, Brufsky AM, et al. Adjuvant therapy with zoledronic acid in patients with breast cancer: a systematic review and meta-analysis. Oncologist. 2013;18(4):353–361. doi: 10.1634/theoncologist.2012-0261. Epub 2013/02/14. PubMed PMID: 23404816; PubMed Central PMCID: PMCPmc3639520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lonning PE, Eikesdal HP. Aromatase inhibition 2013: clinical state of the art and questions that remain to be solved. Endocr Relat Cancer. 2013;20(4):R183–R201. doi: 10.1530/ERC-13-0099. Epub 2013/04/30. PubMed PMID: 23625614; PubMed Central PMCID: PMCPmc3689263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mathew A, Brufsky A. Bisphosphonates in breast cancer. Int J Cancer. 2014 doi: 10.1002/ijc.28965. Epub 2014/05/16. PubMed PMID: 24824552. [DOI] [PubMed] [Google Scholar]
- 31.Ellis GK, Bone HG, Chlebowski R, Paul D, Spadafora S, Fan M, et al. Effect of denosumab on bone mineral density in women receiving adjuvant aromatase inhibitors for non-metastatic breast cancer: subgroup analyses of a phase 3 study. Breast Cancer Res Treat. 2009;118(1):81–87. doi: 10.1007/s10549-009-0352-y. Epub 2009/03/25. PubMed PMID: 19308727. [DOI] [PubMed] [Google Scholar]
- 32.Ellis GK, Bone HG, Chlebowski R, Paul D, Spadafora S, Smith J, et al. Randomized trial of denosumab in patients receiving adjuvant aromatase inhibitors for nonmetastatic breast cancer. J Clin Oncol. 2008;26(30):4875–4882. doi: 10.1200/JCO.2008.16.3832. Epub 2008/08/30. PubMed PMID: 18725648. [DOI] [PubMed] [Google Scholar]
- 33.Greenspan SL, Parker RA, Ferguson L, Rosen HN, Maitland-Ramsey L, Karpf DB. Early changes in biochemical markers of bone turnover predict the long-term response to alendronate therapy in representative elderly women: a randomized clinical trial. J Bone Miner Res. 1998;13:1431–1438. doi: 10.1359/jbmr.1998.13.9.1431. [DOI] [PubMed] [Google Scholar]
- 34.Greenspan SL, Rosen HN, Parker RA. Early changes in serum N-telopeptide and C-telopeptide cross-linked collagen type 1 predict long-term response to alendronate therapy in elderly women. J Clin Endocrinol Metab. 2000;85:3537–3540. doi: 10.1210/jcem.85.10.6911. [DOI] [PubMed] [Google Scholar]