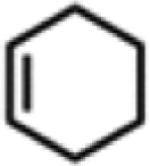
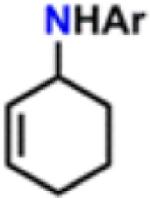
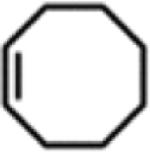
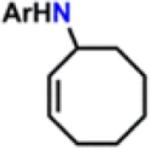
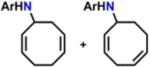
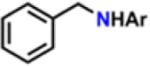
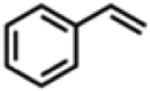
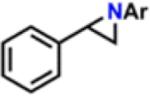
Table 1.
Catalytic Reactivity of 2 and 3,5-Bis(trifluoromethyl)phenyl Azided
Isolated yields.
1H NMR yield using ferrocene as internal standard.
19F NMR yield using 1,2-difluorobenzene as internal standard.
All reactions were conducted neat in substrate.