Abstract
Kv7 (KCNQ) channels, formed as homo- or heterotetramers of Kv7.4 and Kv7.5 α-subunits, are important regulators of vascular smooth muscle cell (VSMC) membrane voltage. Recent studies demonstrate that direct pharmacological modulation of VSMC Kv7 channel activity can influence blood vessel contractility and diameter. However, the physiologic regulation of Kv7 channel activity is still poorly understood. Here, we study the effect of cAMP/protein kinase A (PKA) activation on whole cell K+ currents through endogenous Kv7.5 channels in A7r5 rat aortic smooth muscle cells or through Kv7.4/Kv7.5 heteromeric channels natively expressed in rat mesenteric artery smooth muscle cells. The contributions of specific α-subunits are further dissected using exogenously expressed human Kv7.4 and Kv7.5 homo- or heterotetrameric channels in A7r5 cells. Stimulation of Gαs-coupled β-adrenergic receptors with isoproterenol induced PKA-dependent activation of endogenous Kv7.5 currents in A7r5 cells. The receptor-mediated enhancement of Kv7.5 currents was mimicked by pharmacological agents that increase [cAMP] (forskolin, rolipram, 3-isobutyl-1-methylxanthine, and papaverine) or mimic cAMP (8-bromo-cAMP); the 2- to 4-fold PKA-dependent enhancement of currents was also observed with exogenously expressed Kv7.5 channels. In contrast, exogenously-expressed heterotetrameric Kv7.4/7.5 channels in A7r5 cells or native mesenteric artery smooth muscle Kv7.4/7.5 channels were only modestly enhanced, and homo-tetrameric Kv7.4 channels were insensitive to this regulatory pathway. Correspondingly, proximity ligation assays indicated that isoproterenol induced PKA-dependent phosphorylation of exogenously expressed Kv7.5 channel subunits, but not of Kv7.4 subunits. These results suggest that signal transduction-mediated responsiveness of vascular smooth muscle Kv7 channel subunits to cAMP/PKA activation follows the order of Kv7.5 >> Kv7.4/Kv7.5 > Kv7.4.
Introduction
Voltage-dependent Kv7 potassium channels, encoded by KCNQ genes, are involved in the regulation of cell excitability (Jentsch, 2000; Robbins, 2001). There are five known members of the KCNQ gene family (KCNQ1–KCNQ5), which are expressed in a variety of excitable cells, ranging from neurons (mainly expressing KCNQ2–KCNQ5) and cardiac myocytes (mainly expressing KCNQ1), to smooth muscle cells of different origins (mainly expressing KCNQ1, KCNQ4, and KCNQ5) (Sims et al., 1985; Barhanin et al., 1996; Wang et al., 1998; Kubisch et al., 1999; Lerche et al., 2000; Schroeder et al., 2000; Ohya et al., 2002; Yeung et al., 2007; Mackie et al., 2008; Jepps et al., 2009; McCallum et al., 2009; Brueggemann et al., 2011, 2012; Svalø et al., 2013). The KCNQ1–KCNQ5 gene products (Kv7.1–Kv7.5 proteins) assemble as homo- or heterotetramers to form functional channels (Schwake et al., 2003).
As modulators of cell excitability, Kv7 channels are tightly regulated. Regulatory mechanisms can control the activity of the channels acutely on a rapid time scale (seconds to minutes). Acute suppression of Kv7 channel activity increases cell excitability, whereas augmentation of the Kv7 channel activity decreases excitability (Wickenden, 2002; Brown, 2008). For example, the excitability of neurons is increased when Kv7 channel–mediated neuronal M-currents are suppressed upon Gαq-coupled receptor activation. The mechanisms by which receptor activation leads to suppression of neuronal M-currents include depletion of phosphatidylinositol 4,5-bisphosphate (PIP2), elevation of cytosolic Ca2+ concentration, and protein kinase C (PKC)–dependent phosphorylation of Kv7.2 channel subunits (Delmas and Brown, 2005). In cardiac myocytes, the amplitude of Kv7.1 channel-mediated slow delayed rectifier currents can be increased via the activation of the β-adrenergic receptor (βAR)/Gαs/cAMP/protein kinase A (PKA) pathway (Chen and Kass, 2011). PKA-dependent regulation of the native neuronal M-current has not been reported to date, although the activity of human Kv7.2 channels overexpressed in Xenopus oocytes was reportedly enhanced by PKA-dependent phosphorylation at Ser52 of the N-terminus (Schroeder et al., 1998). Treatment with a membrane-permeant cAMP analog or intracellular application of a PKA catalytic subunit also enhanced human Kv7.4 current in an expression system (Chambard and Ashmore, 2005).
While the functional regulation of the neuronal and cardiac Kv7 channels has been investigated in considerable detail, physiologic regulation of the Kv7 channels in smooth muscles remains sparsely studied. PKC-dependent suppression of native Kv7 currents in response to Gαq-coupled receptor activation was reported for vascular and airway smooth muscle cells, leading to vasoconstriction or bronchoconstriction, respectively (Brueggemann et al., 2007, 2012, 2014c; Mackie et al., 2008; Mani et al., 2013). In terms of smooth muscle relaxation, there is some evidence that endogenous Kv7 channels are downstream targets for βAR-mediated vasodilation. Originally, βAR stimulation was found to enhance M-currents detected in toad gastric smooth muscle cells (Sims et al., 1988). Later, βAR stimulation was reported to enhance Kv7 currents detected in renal artery myocytes (Chadha et al., 2012). In contrast, activation of βARs did not enhance native Kv7 currents in airway myocytes (Brueggemann et al., 2014b), and membrane permeable cAMP or cAMP-elevating agents failed to enhance native Kv7 currents in retinal pigment epithelial cells expressing Kv7.4/7.5 channels (Pattnaik and Hughes, 2012). In the present study, we investigate molecular mechanisms underlying the regulation of vascular Kv7 potassium channels, predominantly formed as homo- or heterotetramers of Kv7.4 and Kv7.5 (Brueggemann et al., 2011, 2014c), by the cAMP/PKA pathway.
Materials and Methods
Constructs.
The adenoviruses to express human KCNQ4 (Adv-hKCNQ4) and human KCNQ5 (Adv-hKCNQ5-FLAG) were created previously using the AdEasy Adenoviral Vector System (Stratagene, La Jolla, CA) (Brueggemann et al., 2011).
Cell Culture.
A7r5 cells were cultured as described previously (Byron and Taylor, 1993). For overexpression studies, A7r5 cells, subcultured at 70% confluence, were infected with Adv-hKCNQ4 or Adv-hKCNQ5-FLAG or both at a multiplicity of infection of 100 and used for electrophysiological experiments 7–10 days after infection. Cells expressing the exogenous channels were identified based on detection of the fluorescence of GFP (coexpressed with the KCNQ products via the IRES-hrGFP element in the Stratagene AdEasy Adenoviral Vector System). To maintain endogenous βAR responsiveness, the cells were serum deprived for 1 to 2 days before use in patch-clamp experiments.
Isolation of Myocytes.
All animal studies were approved by the Loyola University Chicago Institutional Animal Care and Use Committee and were conducted in accordance with the 1996 Guide for the Care and Use of Laboratory Animals of the National Academy of Sciences (Washington, DC). Adult male Sprague-Dawley rats were anesthetized by inhalation of isoflurane, and segments of small intestinal mesentery were surgically removed as described previously (Henderson and Byron, 2007). Methods for isolation of mesenteric artery smooth muscle cells (MASMCs) were described previously (Mackie et al., 2008). Freshly isolated MASMCs were kept on ice until use. The cells were then dispensed onto a glass coverslip base of the recording chamber and allowed to adhere for at least 15 minutes at room temperature.
Patch-Clamp Electrophysiology.
The whole cell perforated patch configuration was used to measure membrane currents under voltage-clamp conditions. All experiments were performed at room temperature with continuous perfusion of bath solution as described previously (Brueggemann et al., 2007, 2011; Mackie et al., 2008). The standard bath solution for A7r5 cells contained (in mM): 5 KCl, 130 NaCl, 10 HEPES, 2 CaCl2, 1.2 MgCl2, 5 D-glucose, pH 7.3. The standard internal (pipette) solution for A7r5 cells contained (in mM): 110 K gluconate, 30 KCl, 5 HEPES, 1 K2EGTA, pH 7.2. Osmolality was adjusted to 268 mOsm/l with D-glucose. The standard bath solution for MASMCs contained (in mM): 140 NaCl, 5.36 KCl, 1.2 MgCl2, 2 CaCl2, 10 HEPES, 10 D-Glucose, pH 7.3, 298 mOsm/l. Standard internal (pipette) solution for MASMCs contained (in mM): 135 KCl, 5 NaCl, 10 HEPES, 0.05 K2EGTA, 1 MgCl2, 20 D-Glucose, pH 7.2, 298 mOsm/l. Amphotericin B (120 μg/ml) was used in the internal solution for membrane patch perforation. To isolate Kv7 currents, 100 μM GdCl3, sufficient to block L- and T-type Ca2+ channels and nonselective cation channels, and to shift activation of 4-AP-sensitive Kv channels to more positive voltages (Mani et al., 2011), was added to external solutions.
Voltage-clamp command voltages were generated using an Axopatch 200B amplifier under control of (Molecular Devices, Sunnyvale, CA) and EPC10 amplifier under control of PATCHMASTER software (HEKA, Pfalz, Germany). Series resistances after amphotericin perforation were 8–15 MΩ and were compensated by 60% in cells overexpressing Kv7 channels. Whole-cell currents were digitized at 2 or 5 kHz and filtered at 1 or 2.9 kHz, respectively.
The K+ currents through overexpressed hKv7 channels were recorded using a 5-second voltage step protocol from a −74 mV holding voltage to test voltages ranging from −114 to −4 mV, followed by a 1-second step to −120 mV. The currents recorded during the last 1 second of recording time for each voltage step were averaged and normalized by cell capacitance to obtain end-pulse steady-state K+ currents. Stable currents were recorded for at least 15 minutes prior to drug application. Time courses of drug effects were recorded by applying 5-second voltage steps to −20 mV every 15 seconds. To measure endogenous currents in A7r5 cells, a 5-second voltage step protocol was used (from a −74 mV holding voltage to test voltages ranging from −94 to +36 mV), followed by a 1-second step to −120 or −30 mV. To analyze the voltage dependence of channel activation the instantaneous tail current amplitude (estimated from an exponential fit of current deactivation measured at −120 mV) was converted to conductance according to the following equation: G = Itail/(−120 − EK), where Itail is the instantaneous tail current amplitude, −120 mV is the tail current step potential, and EK is the reversal potential for potassium (−86 mV). Conductance plots in the absence (control) and in the presence of isoproterenol (1 μM) for each experiment were fitted to a Boltzmann distribution: G(V) = Gmax/[1 + exp(V0.5 − V)/k], where G is conductance, Gmax is a maximal conductance, V0.5 is the voltage of half-maximal activation, and k is the slope factor. Deactivation kinetics were analyzed by applying single exponential fits to the tail currents recorded using a 5-second voltage step protocol (from a −74 mV holding potential to −20 mV), followed by 1-second repolarization to −120 mV.
The Kv7 currents in MASMCs were recorded by application of 5-second voltage steps from a −4 mV holding voltage to test voltages ranging from −84 to +16 mV. Time courses of drug effects on Kv7 currents were recorded at −20 mV holding voltage.
Proximity Ligation Assays (PLAs).
Duolink PLA assays (Sigma-Aldrich, St. Louis, MO) were performed essentially as described previously (Brueggemann et al., 2014c; Tripathi et al., 2015). A7r5 cells infected with Adv-hKCNQ4 or Adv-hKCNQ5-FLAG (Brueggemann et al., 2011) at a multiplicity of infection of 100 were plated on Permanox 8-well tissue culture slides (Nunc, Thermo Fisher Scientific, Waltham, MA). 7–10 days after infection. On the next day, cells were washed with control buffer (5.9 mM KCl, 135 mM NaCl, 10 mM HEPES, 1.5 mM CaCl2, 1.2 mM MgCl2, 11.5 mM glucose, pH 7.3) and treated with vehicle (control buffer) or 1 μM isoproterenol for 5 minutes (a subset of cells was pretreated with 1 μM PKA inhibitor KT5720 for 30 minutes). After treatment, cells were fixed for 15 minutes with 2% paraformaldehyde in phosphate-buffered saline (PBS). The cells were permeabilized with 0.5% Triton X-100 in PBS for 20 minutes at room temperature and then blocked with 3% bovine serum albumin in PBS for 2 hours. Primary mouse antibodies [anti-FLAG antibody (Sigma-Aldrich, at a dilution 1:500) for cells expressing hKv7.5, or anti-KCNQ4 antibodies (Abcam, at dilution 1:500) for cells expressing hKv7.4] were applied in combination with rabbit anti-phospho-(Ser/Thr) PKA substrate (Cell Signaling, at dilution 1:100) in blocking buffer (3% bovine serum albumin in PBS) at 37°C for 2 hours in a humidifying chamber. As an antibody control, slides were incubated with PBS without primary antibody. Slides were then washed and incubated with secondary anti-rabbit/anti-mouse antibodies conjugated with plus/minus Duolink II PLA probes (1:5). After washing, slides were incubated with a ligation-ligase solution to join the adjacent oligonucleotides by ligation into circular DNA molecules (30 minutes, 37°C). This was followed by incubation with amplification-polymerase solution (2 hours, 37°C) following the manufacturer’s protocol. Under these conditions, the circular DNA molecules were amplified by rolling-circle amplification primed by one of the proximity probes, thus creating a concatemeric amplification product that remained covalently attached to the proximity probe (Söderberg et al., 2008). The rolling-circle amplification product was subsequently detected by hybridization of fluorescently labeled oligonucleotides. Slides were mounted with a minimal volume of Duolink II Mounting Medium with 4′,6-diamidino-2-phenylindole for 15–30 minutes and PLA signals (Duolink In Situ Detection Reagents Red; λexcitation/emission 598/634 nm) were identified as fluorescent dots under a Zeiss Axiovert 200M microscope with EC Plan-Neofluor 40×/1.30 oil or EC Plan-Neofluor 100×/1.30 oil objectives and a Zeiss Axio CamMRc5 camera. A series of images (∼8–10/cell) were acquired with z-axis scanning (1 μm z-axis interval) using a wavelet fusion algorithm to combine those images into one single composite z-stack image (Zeiss AxioVision Rel.4.8.2 software). Images were captured and processed identically across all experimental groups. Quantification of PKA-dependent channel phosphorylation (as PLA signals per cell) was performed using the Duolink Image Tool software (Sigma-Aldrich). Images were imported in merged tiff formats containing both signal and nuclei channels. Merged images were checked visually and verified for analytical quality. A total of 21 fields (seven fields from each of three different cell preparations) were analyzed for each Kv7 channel subtype.
Materials.
Cell culture media were from Gibco-BRL (Gaithersburg, MD) or MediaTech (Herndon, VA). Isoproterenol (4-[1-hydroxy-2-[(1-methylethyl)amino]ethyl]-1,2-benzenediol hydrochloride), forskolin ([3R-(3α,4aβ,5β,6β,6aα,10α,10aβ,10bα)]-5-(Acetyloxy)-3-ethenyldodecahydro-6,10,10b-trihydroxy-3,4a,7,7,10a-pentamethyl-1H-naphtho[2,1-b]pyran-1-one), 3-isobutyl-1-methylxanthine (IBMX), papaverine hydrochloride (6,7-dimethoxy-1-veratrylisoquinoline hydrochloride), collagenase, elastase, and Duolink PLA assay reagents were from Sigma-Aldrich. XE-991 (10,10-bis(4-pyridinylmethyl)-9(10H)-anthracenone) dihydrochloride was from Ascent Scientific (Princeton, NJ). Rolipram (4-[3-(Cyclopentyloxy)-4-methoxyphenyl]-2-pyrrolidinone), KT5720 ((9R,10S,12S)-2,3,9,10,11,12-hexahydro-10-hydroxy-9-methyl-1-oxo-9,12-epoxy-1H-diindolo[1,2,3-fg:3′,2′,1′-kl]pyrrolo[3,4-i][1,6]benzodiazocine-10-carboxylic acid, hexyl ester), ML213 (N-(2,4,6-trimethylphenyl)-bicyclo[2.2.1]heptane-2-carboxamide), and 8-bromo-cAMP sodium salt were from Tocris Bioscience (Bristol, United Kingdom). Amphotericin B was from Calbiochem (San Diego, CA). The vector pIRES2-EGFP was from Clontech (Mountain View, CA). The AdEasy Adenoviral Vector System was from Stratagene. The human KCNQ4 cDNA (accession number: AF105202, originally in the insect cell expression vector pMT) was a generous gift from Dr. I. Wood at the University of Leeds (Leeds, United Kingdom). The human KCNQ5 cDNA (accession number: AF202977, originally in the insect cell expression vector pMT) was a generous gift from Dr. T. Jentsch at the Max-Delbrück-Centrum for Molecular Medicine (Berlin, Germany).
Statistics.
Data are expressed as mean ± S.E. SigmaStat (Systat Software, Inc.) was used for all statistical analyses. The paired Student’s t test was used for comparisons of parameters measured before and after treatments. Comparisons among multiple treatment groups were evaluated by analysis of variance followed by a Holm-Sidak post hoc test or analysis of variance on ranks followed by multiple comparisons versus control group (Dunn’s method). Differences associated with two-tailed P values < 0.05 were considered statistically significant.
Results
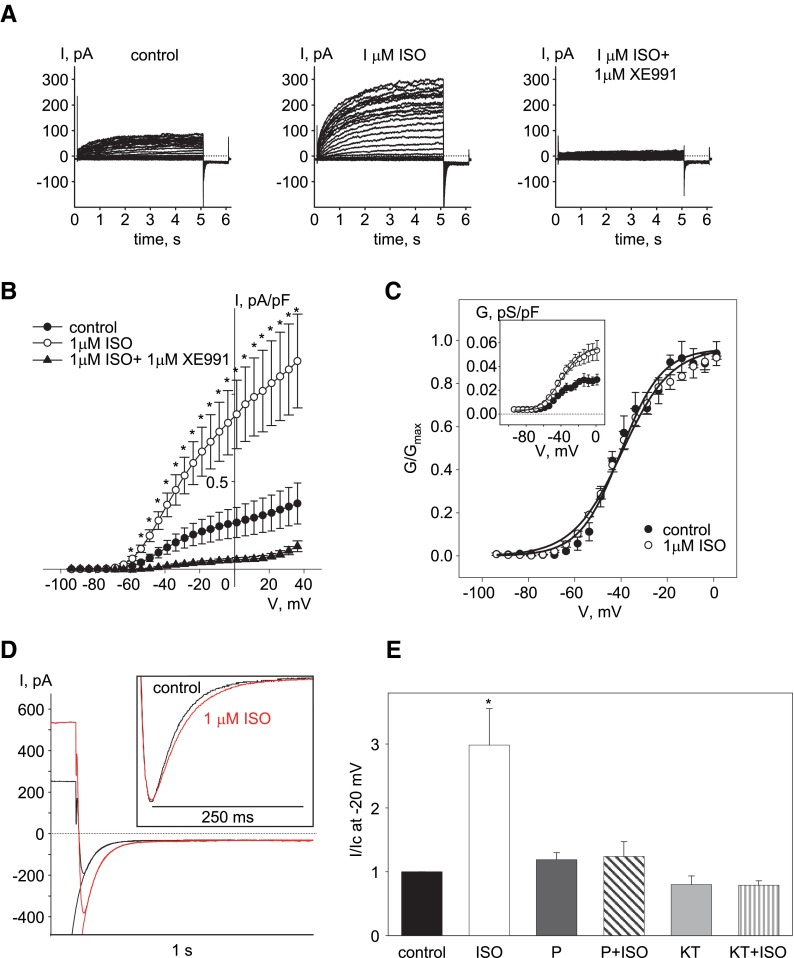
We previously used the A7r5 embryonic rat aortic cell line as a model system to investigate the regulation of native Kv7.5 channels and as an expression system for functional vascular Kv7.4, Kv7.5, and Kv7.4/7.5 channels (Brueggemann et al., 2007, 2011, 2014c). Evidence for the presence of functional native Kv7.5 channels in A7r5 cells as a sole source of conductance in the voltage range from −60 to +20 mV under the recording conditions used here were obtained previously based on reverse transcription polymerase chain reaction (Brueggemann et al., 2007, 2011), pharmacology (Brueggemann et al., 2007, 2011), molecular approaches using shRNA (Brueggemann et al., 2007; Mani et al., 2009), and abolishment of the current upon expression of the dominant negative Kv7.5 subunit (L.I. Brueggemann and K.L. Byron, unpublished results). A7r5 cells have also been reported to express endogenous βARs (Hirata et al., 1985). When we applied isoproterenol (1 μM, to activate endogenous βARs), it induced a 3-fold increase in the amplitude of endogenous Kv7.5 currents in serum-deprived A7r5 cells. The isoproterenol-induced increase in current amplitude was abolished by coapplication of a pan Kv7 channel blocker, XE991 (1 μM) (Fig. 1, A, B). The isoproterenol effect was also prevented by propanolol (10 μM), a βAR antagonist, or by pretreatment with a PKA inhibitor KT5720 (1 μM for 30 minutes) (Fig. 1E). Isoproterenol induced neither a shift in voltage dependence of activation nor a change in the slope of the conductance plot of native Kv7.5 channels (Fig. 1C; Table 1); however, it did slightly decrease the deactivation rate of endogenous Kv7.5 current measured at −120 mV, from 40.3 ± 0.9 to 49.7± 3.3 ms (P < 0.05, n = 4, paired Student’s t test) (Fig. 1D).
Fig. 1.
Enhancement of endogenous Kv7.5 current in A7r5 cells by isoproterenol depends on activation of endogenous βARs and PKA. (A) Representative traces of endogenous Kv7.5 currents recorded in an A7r5 cell before (control, left panel), after 5 minutes in the presence of 1 μM isoproterenol (middle panel), and following addition of 1 μM XE991 (right panel) (C = 151 pF, representative of 11 similar experiments). (B) Current-voltage relationships of endogenous Kv7.5 current densities recorded in A7r5 cells before (control, solid circles), after 5 minutes of treatment with 1 μM isoproterenol (open circles), and after 10 minutes of treatment with 1 μM isoproterenol in combination with 1 μM XE991 (solid triangles). The asterisks (*) indicate significant difference from control, P < 0.05, one-way repeated-measures analysis of variance (ANOVA), n = 5–11. (C) Conductance-voltage relationships of endogenous Kv7.5 channels normalized to maximal conductance (Gmax) before (solid circles, n = 5) and after addition of 1 μM isoproterenol (open circles, n = 5) fitted to the Boltzmann equation (solid lines). Absolute values of conductance densities are shown in the inset. (D) Deactivation tail currents recorded at −120 mV before (black) and after addition of 1 μM isoproterenol (red), fitted by a single exponential function (solid lines). Endogenous Kv7.5 channels were activated by 5-second steps to −20 mV, followed by a 1-second step to −120 mV; five traces were averaged for each condition. The inset shows tail currents normalized to peak amplitude in order to highlight the differences in deactivation kinetics. (E) Normalized currents recorded at −20 mV in control (black bar) and in the presence of the following: 1 μM isoproterenol (ISO, open bar, n = 5); 10 μM propranolol (P, dark gray bar, n = 4); 10 μM propranolol plus 1 μM isoproterenol (P + ISO, dark gray striped bar, n = 4); 1 μM KT5720 (KT, light gray bar, n = 4); and 1 μM KT5720 plus 1 μM isoproterenol (KT + ISO, light gray striped bar, n = 4). The asterisks (*) indicate significant difference from control (P < 0.05, one-way ANOVA on ranks).
TABLE 1.
Effect of 1 μM isoproterenol on the voltage dependence of endogenous Kv7.5 and exogenous homomeric hKv7.4, homomeric hKv7.5, and heteromeric hKv7.4/7.5 channels
| Kv7 isoform |
Gmax ISO/Gmax Control x 100% |
V0.5 |
k |
||
|---|---|---|---|---|---|
| Control |
1 μM ISO |
Control |
1 μM ISO |
||
| % | mV | mV | mV | mV | |
| Endogenous Kv7.5, n = 10 | 259.3 ± 33.8a | −48.5 ± 2.5 | −47.8 ± 2.7 | 9.5 ± 1.0 | 11.3 ± 0.8 |
| Exogenous hKv7.5, n = 5 | 267.4 ± 16.6b | −46.2 ± 2.5 | −46.1 ± 2.1 | 12.3 ± 0.8 | 13.6 ± 1.7 |
| Exogenous hKv7.4, n = 5 | 98.1 ± 3.1 | −28.6 ± 2.0 | −28.2 ± 1.6 | 10.8 ± 0.7 | 10.7 ± 0.7 |
| Exogenous hKv7.4/7.5, n = 5 | 134.9 ± 9.3a | −34.0 ± 3.9 | −35.5 ± 5.6 | 11.3 ± 0.8 | 12.1 ± 0.9 |
Gmax, maximal conductance; ISO, isoproterenol; k, slope factor; V0.5, voltage of half-maximal activation.
Significantly different from control, paired Student’s t test, P < 0.05.
Significantly different from control, paired Student’s t test, P < 0.001.
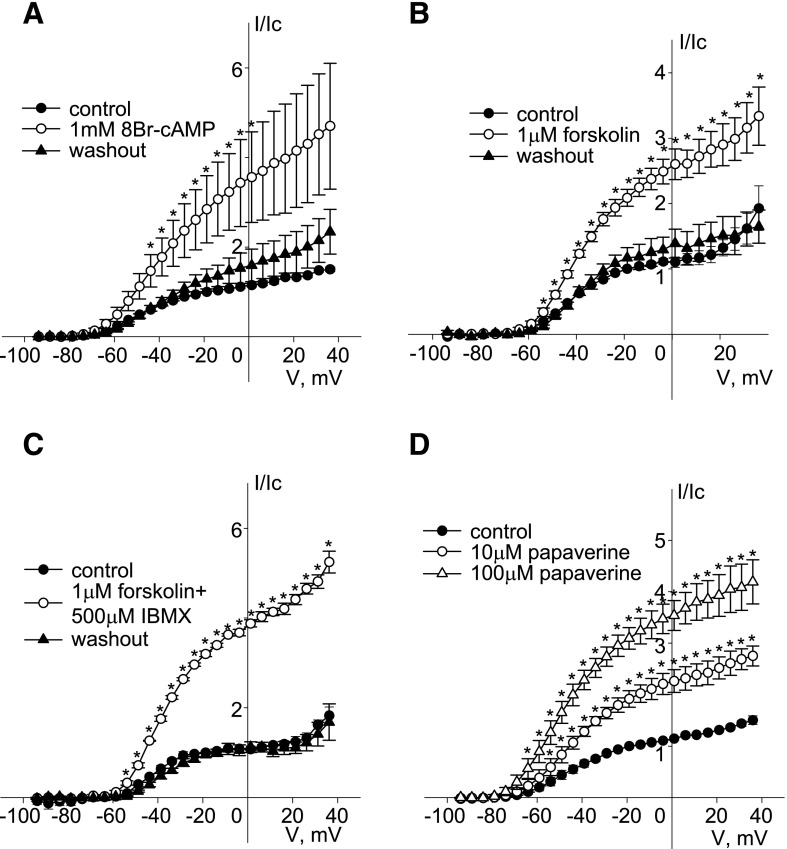
Application of a membrane permeant cAMP analog, 8-bromo cAMP (1 mM) also reversibly increased the amplitude of endogenous Kv7.5 currents by 2- to 3-fold in the voltage range from −54 to +36 mV (n = 4) (Fig. 2A). A similar reversible enhancement of endogenous Kv7.5 currents in A7r5 cells was observed upon activation of endogenous adenylate cyclase with forskolin (1 μM; >2-fold increase in amplitude in the voltage range positive to −54 mV; n = 6) (Fig. 2B). It is worth noting that when the concentration of forskolin was increased from 1 to 10 μM, enhancement of endogenous Kv7.5 was reversed to almost complete inhibition (data not shown). When activation of adenylate cyclase by forskolin (1 μM) was combined with inhibition of phosphodiesterases (PDEs) by addition of the nonselective PDE inhibitor 3-isobutyl-1-methylxanthine (IBMX, 500 μM), endogenous Kv7.5 currents were enhanced >3-fold in the voltage range from −54 to +36 mV (n = 4) (Fig. 2C). Another nonselective PDE inhibitor, papaverine, dose dependently enhanced endogenous Kv7.5 currents by ∼2- and ∼3-fold at doses of 10 and 100 μM, respectively, even in the absence of forskolin (Fig. 2D).
Fig. 2.
Enhancement of endogenous Kv7.5 current in A7r5 cells by elevation of intracellular [cAMP]. (A) Current-voltage (I-V) relationships of endogenous Kv7.5 currents recorded in A7r5 cells before (control, solid circles), after 15 minutes of treatment with 1 mM 8-bromo cAMP (8Br-cAMP) (open circles), and after 10 minutes of washout (solid triangles). Currents were normalized to currents recorded at −20 mV before application of 8Br-cAMP [n = 4, * indicates significant difference from control, P < 0.05, one-way repeated-measures analysis of variance (ANOVA)]. (B) I-V relationships of endogenous Kv7.5 currents recorded in A7r5 cells before (control, solid circles), after 5 minutes of treatment with 1 μM forskolin (open circles), and after 10 minutes of washout (solid triangles). Currents were normalized to currents recorded at −20 mV before application of forskolin (n = 6, * indicates significant difference from control, P < 0.05, one-way repeated-measures ANOVA). (C) I-V relationships of endogenous Kv7.5 currents recorded in A7r5 cells before (control, solid circles), after 5 minutes of treatment with 1 μM forskolin in the presence of 500 μM IBMX (open circles), and after 10 minutes of washout (solid triangles). Currents were normalized to currents recorded at −20 mV before application of forskolin/IBMX (n = 4, * indicates significant difference from control, P < 0.05, one-way repeated-measures ANOVA). (D) I-V relationships of endogenous Kv7.5 currents recorded in A7r5 cells before (control, solid circles) and after treatment with papaverine (10 μM open circles, 100 μM open triangles). Currents were normalized to currents recorded at −20 mV before application of papaverine (n = 4, * indicates significant difference from control, P < 0.01, one-way repeated-measures ANOVA).
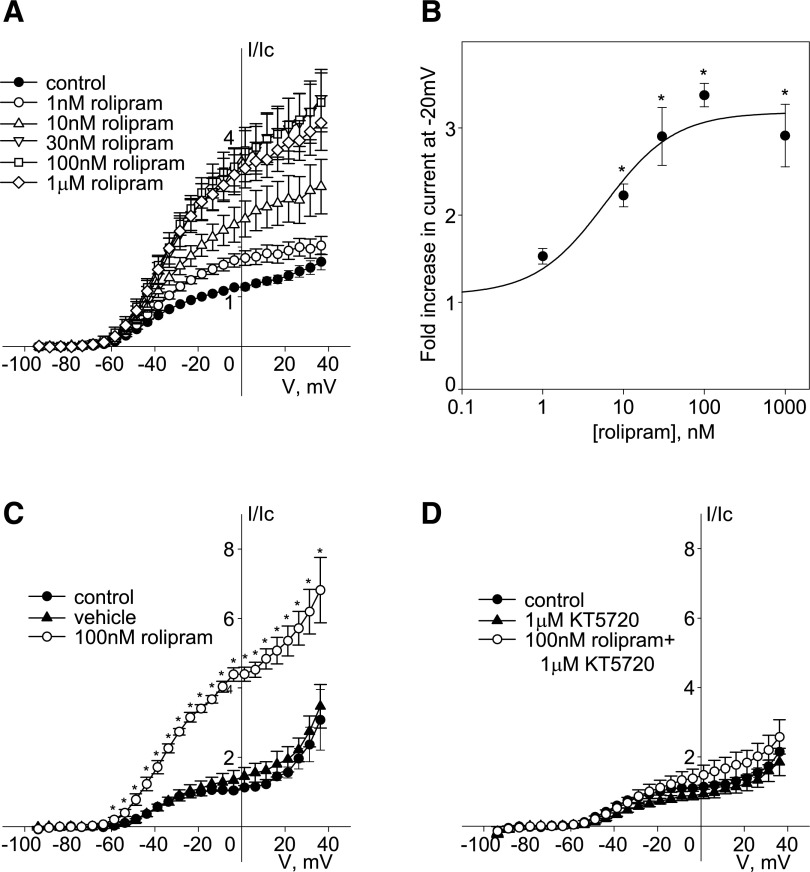
The more selective inhibitor of PDE4 (cAMP PDE isoform), rolipram, applied at increasing concentrations (1 nM–1 μM), dose dependently and reversibly enhanced endogenous Kv7.5 current in A7r5 cells, with an EC50 ∼6 nM and maximal enhancement (2.1-fold ± 0.5-fold) achieved at 100 nM (Fig. 3, A and B). To test whether PDE4 inhibition by rolipram enhances endogenous Kv7.5 currents via activation of PKA, we used the PKA inhibitor KT5720 (1 μM). Pretreatment with KT5720 (1 μM for 30 minutes) significantly inhibited the effect of 100 nM rolipram, relative to vehicle pretreatment (Fig. 3, C and D).
Fig. 3.
Inhibition of PDE4 with rolipram enhances endogenous Kv7.5 current in A7r5 cells in a PKA-dependent manner. (A) Current-voltage (I-V) relationships of endogenous Kv7.5 currents recorded in A7r5 cells before (control, solid circles) and during treatment with increasing concentrations of rolipram (open symbols: circle, 1 nM; triangle, 10 nM; reversed triangle, 30 nM; square, 100 nM; and diamond, 1 μM). (B) Cumulative rolipram dose-response curve for Kv7.5 current enhancement, presented as fold increase in amplitude of current recorded at −20 mV, fitted to the Hill equation [n = 4–5, * indicates significant difference from control, P < 0.001, one-way repeated-measures analysis of variance (ANOVA)]. (C and D) I-V relationships of Kv7.5 currents recorded in A7r5 cells before (control, solid circles), after 30 minutes of pretreatment with either vehicle for KT5720 (0.01% dimethylsulfoxide, solid triangles) (C) or 1 μM KT5720 (D), and after 5 minutes of treatment with 100 nM rolipram (open circles). Currents were normalized to currents recorded at −20 mV before application of rolipram (n = 4, * indicates significant difference from control, P < 0.05, one-way repeated-measures ANOVA).
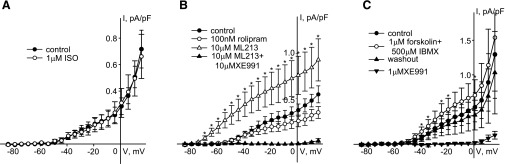
Freshly isolated rat MASMCs differ from A7r5 cells in that they express both Kv7.4 and Kv7.5 subunits, which predominantly form heteromeric channels (Brueggemann et al., 2011, 2014c), whereas A7r5 cells only express Kv7.5 (Brueggemann et al., 2007, 2011). We previously found that PKC-dependent regulation of heteromeric Kv7.4/7.5 channels in MASMCs differs from the regulation of homomeric Kv7.5 channels in A7r5 cells (Brueggemann et al., 2014c). In the present study, the same was found to be true for PKA-dependent regulation. Direct activation of βARs in MASMCs by isoproterenol (1 μM) did not significantly enhance endogenous Kv7 current (n = 5) (Fig. 4A). Similarly, rolipram (100 nM) also failed to enhance endogenous Kv7 current in MASMCs (Fig. 4B); a slight suppression of the current observed in the presence of rolipram was not statistically significant. Subsequent application of the nonselective Kv7.2–Kv7.5 activator ML213 (10 μM) (Yu et al., 2011; Brueggemann et al., 2014a) robustly enhanced the MASMC Kv7 currents and this effect was reversed by the selective Kv7 channel blocker XE991 (10 μM) (Fig. 4B), demonstrating the expected pharmacological characteristics of the Kv7 currents and confirming our ability to detect an increase in current amplitude in these cells. Only direct activation of adenylate cyclase with forskolin (1 μM) in combination with the nonselective PDE inhibitor IBMX (500 μM) induced a modest enhancement of Kv7 currents in MASMCs, by 66% ± 9% in the voltage range from −39 to −19 mV (Fig. 4C), in comparison with the 3.5-fold enhancement of endogenous Kv7.5 current in A7r5 cells with the same treatment (Fig. 2C).
Fig. 4.
Regulation of endogenous Kv7 currents in mesenteric artery myocytes by isoproterenol, rolipram, and forskolin/IBMX. (A) Current-voltage (I-V) relationships of endogenous Kv7 currents recorded in MASMCs before (control, solid circles) and after 5 minute treatment with 1 μM isoproterenol (open circles, n = 6). (B) I-V relationships of endogenous Kv7 currents recorded in MASMCs before (control, solid circles) and after 5 minutes of treatment with 100 nM rolipram (open circles, n = 5), followed by application of ML213 (10 μM, open triangles, n = 5) and 10 μM XE991 in the presence of 10 μM ML213 (solid triangles, n = 4). The asterisks (*) indicate significant difference from control, P < 0.05, one-way repeated-measures analysis of variance. (C) I-V relationships of endogenous Kv7 currents recorded in MASMCs before (control, solid circles) and after 5 minutes of treatment with 1 μM forskolin in the presence of 500 μM IBMX (open circles, n = 5, * indicates significant difference from control, P < 0.05, paired Student’s t test) and after 10 minutes of washout (solid upward triangles), followed by application of 1 μM XE991 (solid downward triangles, n = 4).
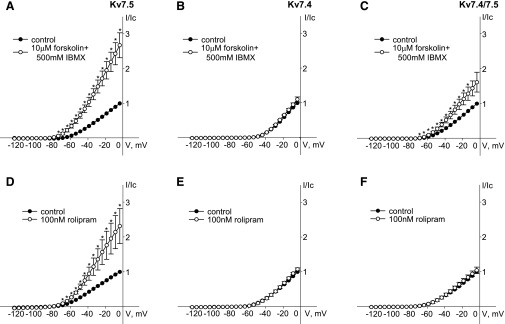
To more directly test the ability of PKA to regulate individual vascular Kv7 channel isoforms we turned back to the A7r5 cell line, using it as an expression system. Human Kv7.4 and Kv7.5 were expressed alone or together to form homomeric Kv7.4, homomeric Kv7.5, or heteromeric Kv7.4/7.5 channels. Consistent with previous studies (Brueggemann et al., 2011, 2014c), the current densities following overexpression of the exogenous Kv7 channel subunits were approximately 100- to 200-fold higher than the average native Kv7.5 current density in A7r5 cells (data not shown), allowing evaluation of effects on the exogenous currents with little contamination of endogenous currents. Both rolipram (100 nM) and the combination of forskolin (10 μM) with IBMX (500 μM) enhanced currents through exogenous Kv7.5 channels by ∼2.5-fold (Fig. 5, A and D). On the other hand, neither rolipram (100 nM) nor the combination of forskolin (10 μM) with IBMX (500 μM) enhanced currents through exogenous Kv7.4 channels (Fig. 5, B and E). Heteromeric Kv7.4/7.5 channels in A7r5 cells responded to application of rolipram and forskolin/IBMX similarly to endogenous Kv7 current in MASMCs; rolipram had no effect on Kv7.4/7.5 currents, while forskolin/IBMX (10 μM/500 μM) induced a moderate enhancement of these currents (by 74% ± 4%) (Fig. 5, C and F).
Fig. 5.
Differential regulation of hKv7.5, hKv7.4, and hKv7.4/7.5 by forskolin/IBMX and rolipram. (A and D) Current-voltage (I-V) curves of steady-state Kv currents recorded in A7r5 cells overexpressing hKv7.5 before (control, solid circles) and after 5 minutes of treatment with 10 μM forskolin in the presence of 500 μM IBMX (A, open circles, n = 7) or 100 nM rolipram (D, open circles, n = 7). (B and E) I-V curves of steady-state Kv currents recorded in A7r5 cells overexpressing hKv7.4 before (control, solid circles) and after 5 minutes of treatment with 10 μM forskolin in the presence of 500 μM IBMX (B, open circles, n = 3) or 100 nM rolipram (E, open circles, n = 5). (C and F) I-V curves of steady-state Kv currents recorded in A7r5 cells overexpressing hKv7.4/7.5 before (control, solid circles) and after 5 minutes of treatment with 10 μM forskolin in the presence of 500 μM IBMX (C, open circles, n = 6) or 100 nM rolipram (F, open circles, n = 5). The asterisks (*) indicate significant difference from control, P < 0.05, paired Student’s t test.
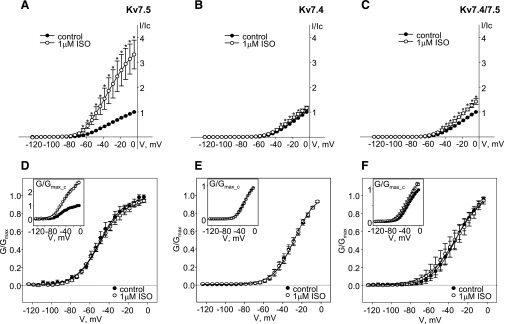
Activation of endogenous βARs with isoproterenol (1 μM) also enhanced exogenous human Kv7.5 homomeric channels by ∼3.5-fold over the voltage range positive to −65 mV (n = 6) (Fig. 6A). In contrast, isoproterenol (1 μM) only very slightly (but significantly) enhanced currents through exogenous Kv7.4 channels at voltages between −34 and −14 mV (n = 6) (Fig. 6B). A slightly greater enhancement was observed for the current through heteromeric Kv7.4/7.5 channels (∼50% enhancement; current amplitudes were significantly greater than control in the voltage range between −44 and −4 mV, n = 5) (Fig. 6C). Similar to the finding for endogenous Kv7.5 channels, there were no changes in the voltage dependence of activation or slopes of conductance plots for exogenous Kv7 channels in the presence of isoproterenol (Fig. 6, D–F; Table 1). Application of isoproterenol (1 μM) slightly decreased the deactivation rate of exogenous Kv7.5 current measured at −120 mV, from 37.3 ± 7.1 to 43.0 ± 7.5 ms (P < 0.05, n = 6, paired Student’s t test).
Fig. 6.
Activation of endogenous βARs in A7r5 cells enhances exogenous hKv7.5, hKv7.4 and hKv7.4/7.5 to varying degrees. (A–C) Current-voltage curves of steady-state Kv currents recorded in A7r5 cells overexpressing hKv7.5 (A, n = 6), hKv7.4 (B, n = 6), or hKv7.4/7.5 (C, n = 5) before (control, solid circles) and after 5 minutes of treatment with 1 μM isoproterenol (open circles). The asterisks (*) indicate significant difference from control, P < 0.05, paired Student’s t test. (D–F) Conductance-voltage relationships of exogenous hKv7.5 (D), Kv7.4 (E), and Kv7.4/7.5 (F) channels normalized to maximal conductance (Gmax) before (solid circles, n = 5-6) and after addition of 1 μM isoproterenol (open circles, n = 5–6) fitted to the Boltzmann equation (solid lines); conductances normalized to the maximal control conductance (Gmax_c) are shown in the insets.
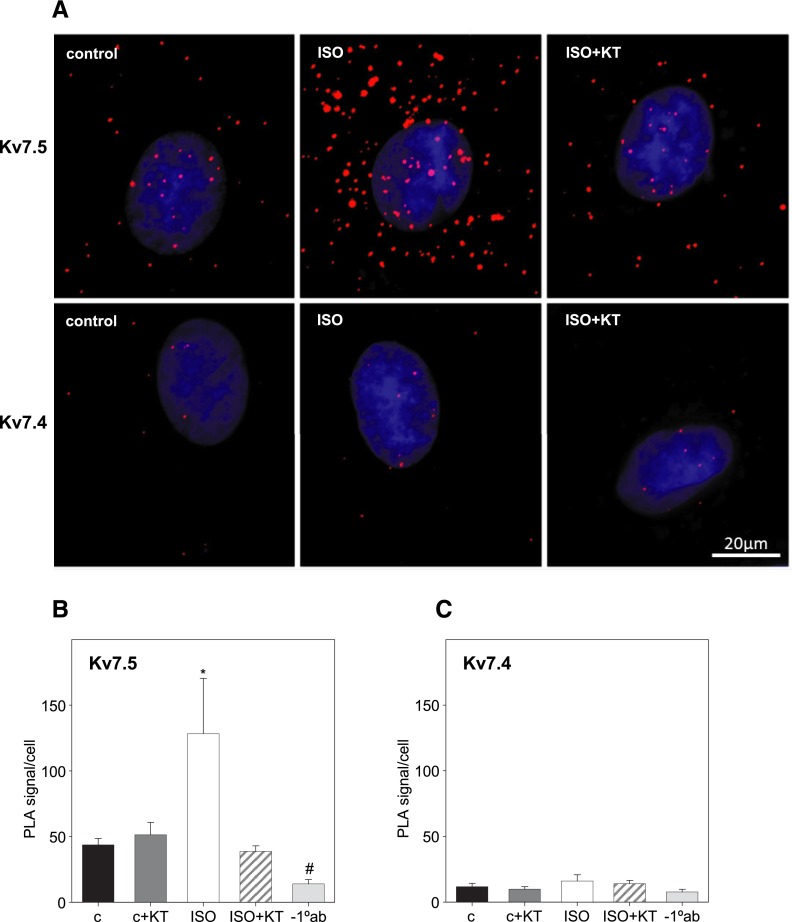
To determine whether activation of endogenous β–adrenergic receptors by isoproterenol results in PKA-dependent phosphorylation of the Kv7.5 and Kv7.4 channel subunits we used PLAs to visualize and quantify channel phosphorylation at single molecule resolution. A7r5 cells overexpressing FLAG-tagged hKv7.5 or hKv7.4 were treated with 1 μM isoproterenol for 5 minutes (with or without 30 minutes of pretreatment with 1 μM KT5720), fixed, and stained with primary antibodies raised in rabbit against phospho-Ser/Thr PKA substrate and with antibodies raised in mouse against FLAG (for FLAG-tagged hKv7.5) or against amino acids 2–77 of human Kv7.4 (for hKv7.4). Isoproterenol (1 μM) induced a significant increase in the number of punctal fluorescent PLA signals in A7r5 cells expressing FLAG-tagged hKv7.5 channels; this effect was abolished by pretreatment with the PKA inhibitor KT5720 (1 μM; Fig. 7). No such increase in PLA signals was observed in A7r5 cells expressing hKv7.4 (Fig. 7), although the antibody against Kv7.4 had been validated for detection of these channels in a PLA assay in a previous study (Brueggemann et al., 2014c).
Fig. 7.
Activation of endogenous βARs in A7r5 cells enhances PKA-dependent phosphorylation of exogenous hKv7.5 but not hKv7.4 channels. (A) Representative images of A7r5 cells exogenously expressing hKv7.5 channels (top panels) and hKv7.4 channels (bottom panels); untreated (control), treated with 1 μM isoproterenol (ISO) for 5 minutes, and treated with 1 μM ISO for 5 minutes after pretreatment with 1 μM KT5720 for 30 minutes (ISO + KT). PLAs were conducted on hKv7.5-expressing cells labeled with a combination of mouse anti-FLAG antibody and rabbit anti-phospho-Ser/Thr PKA substrate antibody or on hKv7.4-expressing cells labeled with a combination of mouse anti-KCNQ4 antibody and rabbit anti-phospho-Ser/Thr PKA substrate antibody. (B and C) Bar graphs summarizing the number of PLA signals/cell in A7r5 cells exogenously expressing hKv7.5 (B) and hKv7.4 (C) under control conditions (c), after 30 minutes of treatment with 1 μM KT5720 (c + KT), after 5 minutes of treatment with 1 μM ISO in the absence (ISO) or presence of pretreatment with 1 μM KT5720 (ISO + KT), and in cells where the primary antibodies were omitted (−1°ab); * and #, P < 0.001 from all group analyses of variance on ranks, n = 12–21.
Discussion
The results of the present study reveal clear differences in the regulation of vascular smooth muscle cell (VSMC) Kv7 channel subtypes by the βAR-cAMP/PKA pathway. Kv7.5 channels are robustly enhanced by stimuli, including βAR activation, that elevate cAMP levels; this is a reversible effect that appears to be dependent on the activation of PKA and direct phosphorylation of the channel subunits. In contrast, Kv7.4 channels are remarkably insensitive to the same treatments. Coexpression of Kv7.4 with Kv7.5, either exogenously (via expression vectors) or when they are natively coexpressed, as in MASMCs, results in heteromeric Kv7.4/Kv7.5 channels that are very weakly sensitive to an elevation of [cAMP]. These differences in response may have important ramifications in terms of vascular reactivity since expression patterns among Kv7 channel subtypes may differ in different vascular beds or they may change during development or with disease.
Elevation of cytosolic [cAMP] in VSMCs, by activation of cell surface receptors or by inhibition of PDEs, is well known to induce vasodilation. Multiple mechanisms for cAMP-mediated vasodilatory responses have been proposed (Zhao et al., 1998; Yang et al., 1999; Maurice et al., 2003; Morgado et al., 2012; Cuiñas et al., 2013). Our findings add activation of Kv7.5 channels as another potential mechanism. Mackie et al. (2008) previously demonstrated that activation of Kv7 channels is sufficient to induce dilation of rat mesenteric arteries. We predict that cAMP/PKA-mediated activation of Kv7.5 currents in VSMCs would reverse or oppose further membrane depolarization and thereby decrease the open probability of voltage-sensitive Ca2+ channels, resulting in decreased Ca2+ entry and decreased contractility. This prediction is supported by previous evidence that forskolin- and isoproterenol-induced vasorelaxation was reduced in the presence of linopirdine, a selective Kv7 channel blocker (Chadha et al., 2012; Lee et al., 2015).
Our findings suggest that inhibition of PDE is sufficient to activate the cAMP/PKA/Kv7.5 channel signaling pathway in A7r5 cells since several PDE inhibitors (rolipram, papaverine, and IBMX) were found to robustly enhance Kv7.5 currents in these cells. Of the known members of the heterogeneous PDE superfamily (PDE1–PDE11), PDE1, PDE3, PDE4, and PDE5 are the predominant enzyme families expressed in vascular myocytes, with PDE3 and PDE4 accounting for the majority of the cAMP-hydrolyzing activity (Polson and Strada, 1996; Maurice et al., 2003). Rolipram, a selective PDE4 inhibitor (Ahmad et al., 2015), robustly activated Kv7 currents in A7r5 cells (Fig. 3) but not in MASMCs (Fig. 4). A7r5 cells natively express only Kv7.5 channels (Brueggemann et al., 2007, 2011), whereas MASMCs predominantly express Kv7.4/Kv7.5 channels (Brueggemann et al., 2011, 2014a). Insensitivity of MASMC Kv7.4/7.5 channels to rolipram was replicated when this same subunit combination was expressed in A7r5 cells, suggesting that the difference in regulation of native Kv7 currents between A7r5 cells and MASMCs relates primarily to the Kv7 channel subunit stoichiometry rather than to differences in PDE isoforms (Polson and Strada, 1996; Dunkerley et al., 2002; Maurice et al., 2003).
IBMX, in combination with the direct activator of adenylate cyclase, forskolin, induced a modest enhancement of currents through Kv7.4/Kv7.5 channels in MASMCs, and through exogenously expressed hKv7.4/Kv7.5 channels in A7r5 cells; however, rolipram failed to elicit a response (Figs. 4 and 5). The pan inhibitor of PDEs, IBMX, may increase [cAMP] to a greater extent (particularly when it is combined with forskolin), and thus induce a greater enhancement of Kv7.4/7.5 currents. We cannot rule out a potential role of cGMP, which may be elevated via the inhibition of PDE5 by IBMX. IBMX and other methylxanthine PDE inhibitors also have a number of documented off-target effects (Wells and Kramer, 1981), which might account for the differences between IBMX and rolipram. In general, our results suggest that modest elevation of cytosolic [cAMP] is sufficient to open Kv7.5 homomeric channels, whereas a greater elevation of cAMP levels is required to open Kv7.4/Kv7.5 heteromeric channels, and Kv7.4 homomeric channels are insensitive to the cAMP/PKA pathway.
A mechanism that could account for the difference in enhancement of Kv7.5 versus Kv7.4 channels in response to cAMP/PKA activation would be the presence of consensus site(s) for PKA-mediated phosphorylation in Kv7.5, but not in Kv7.4 channels. The Kv7.5 channel contains one reported PKA phosphorylation site (Schroeder et al., 2000). However, evaluation of amino acid sequences using MIT Scansite software (Obenauer et al., 2003) revealed 11 putative PKA phosphorylation sites in Kv7.5, only two of which have homologous residues in Kv7.4. Using PLAs, we detected an increase in PKA-dependent phosphorylation of Kv7.5 in response to activation of βARs with isoproterenol (Fig. 7); however, it has yet to be determined which sites might be phosphorylated to elicit the increase in current amplitude that we observed in the present study. We failed to detect PKA-dependent phosphorylation of Kv7.4 channel subunits, supporting the possibility that the Kv7.5 and Kv7.4 subunits may be differentially phosphorylated, and hence have different sensitivities to PKA activation.
The mechanism by which PKA-dependent phosphorylation of Kv7.5 channel subunits enhances current amplitude remains unknown. Thus far, the only physiologic mechanism proposed for positively regulating the activity of Kv7 channels is an increase of membrane concentration of PIP2 (Suh and Hille, 2007). PIP2 is a minor membrane phospholipid that associates with Kv7 channels and stabilizes their open state (Li et al., 2005). PKA-dependent phosphorylation of the Kv7.1 channels was suggested to increase apparent affinity of the channel to PIP2 (Lopes et al., 2007). Considering that the isoproterenol-induced change of conductance-voltage relationships of Kv7.5 channels is similar to the effect of increased PIP2 concentration on open probability of Kv7 channels (no shifts or changes of slope of conductance plots) (Zaydman and Cui, 2014), we speculate that PKA-dependent phosphorylation of the Kv7.5 channel α-subunits increases their apparent PIP2 affinity and thus increases channel open probability.
At the mRNA level, almost all vascular myocytes tested have shown predominant expression of KCNQ4 transcripts, followed by KCNQ1 and KCNQ5 (Yeung et al., 2007; Joshi et al., 2009; Zhong et al., 2010; Ng et al., 2011; Chadha et al., 2012). However, functional assembly of Kv7 channel protein subunits within vascular myocytes is just beginning to be understood. Use of biochemical methods, including PLAs, fluorescence resonance energy transfer, and coimmunoprecipitation, has suggested the existence of Kv7.4/Kv7.5 heterotetramers in mesenteric and cerebral artery myocytes as well as Kv7.1/Kv7.5 heterotetramers in aortic myocytes (Brueggemann et al., 2014c; Chadha et al., 2014; Oliveras et al., 2014). Pharmacological and siRNA knockdown approaches also suggest that functional channels in mesenteric and cerebral artery myocytes are predominantly Kv7.4/Kv7.5 heterotetramers, rather than Kv7.4 or Kv7.5 homotetramers (Brueggemann et al., 2011; Chadha et al., 2014). Our results suggest that activation of vascular Kv7.4/7.5 channels by stimulation of βARs would only modestly increase outward currents (e.g., Fig. 6C), thus this mechanism would therefore be expected to be a minor contributor to vasodilation. However, to the extent that some vascular beds may express Kv7.5 homomeric channels, there remains some potential for βAR-mediated vasodilation via this pathway.
Systemic and pulmonary hypertensive conditions have been reported to be associated with a reduction in KCNQ4 mRNA and Kv7.4 protein in arterial myocytes, while KCNQ5 mRNA levels were unaltered (Chadha et al., 2012; Khanamiri et al., 2013; Sedivy et al., 2015). The selective loss of Kv7.4 subunits would likely shift the stoichiometry of the functional Kv7 channels toward a predominantly Kv7.5 channel phenotype. Therefore, in hypertensive states, which are typically associated with increased sympathetic drive, the βAR-cAMP/PKA–mediated activation of Kv7.5 channels in myocytes described here (Figs. 1, 2, and 6) would play a protective role in preventing a hypercontracted state of vascular myocytes. This interpretation is lent support by the result that coronary artery preparations that exhibited higher expression of KCNQ5 mRNA/protein relaxed more in response to forskolin than artery preparations containing less KCNQ5, when KCNQ4 mRNA levels were comparable between them (Morales-Cano et al., 2015).
We previously found that PKC-dependent phosphorylation and suppression of Kv7 activity by arginine vasopressin (acting through Gαq-coupled receptors on A7r5 cells) were dependent on the subunit composition of the channels (Brueggemann et al., 2014c). Overexpressed hKv7.5 channels in A7r5 cells were found to be highly sensitive to this PKC-dependent suppression of channel activity; however, as in the present study, Kv7.4 homomeric channels were resistant to this form of regulation, while heteromeric Kv7.4/7.5 channels displayed intermediate sensitivity (Brueggemann et al., 2014c).
It is worth noting that the A7r5 cell line is derived from embryonic rat thoracic aorta (Kimes and Brandt, 1976); the robust expression of KCNQ5 with no detectable KCNQ4 (Brueggemann et al., 2007; 2011) may relate to the developmental stage of the tissue from which these cells were isolated. As noted previously, vascular myocytes from adult arteries have almost uniformly been found to express higher levels of KCNQ4 than KCNQ5. Although no studies have been conducted to examine the developmental changes in KCNQ gene expression or Kv7 channel function in the vasculature, it is intriguing to speculate that developmental changes in Kv7 channel subunit stoichiometry might be associated with differences in channel sensitivities to regulatory pathways.
The findings of the present study are consistent with previous research in implicating Kv7.5 subunits as the primary regulatory target in Kv7 channels of VSMCs. To the extent that these subunits are predominant, as in the embryonic rat aorta–derived A7r5 cells, the channels formed are highly responsive to both positive (cAMP/PKA) and negative (PKC) regulatory pathways. To the extent that Kv7.4 subunits are coexpressed, as in MASMCs from adult rats or the more extreme case when human KCNQ4 is overexpressed in A7r5 cells, the channels become progressively less responsive. Future studies will determine whether changing expression of Kv7 channel subtypes, as may occur during development or in pathologies such as hypertension, results in altered Kv7 channel–dependent vascular responsiveness. This could provide important clues for designing appropriate Kv7 channel–targeted therapeutic regimens to treat cardiovascular diseases.
Abbreviations
- ANOVA
analysis of variance
- βAR
β-adrenergic receptor
- IBMX
3-isobutyl-1-methylxanthine
- MASMC
mesenteric artery smooth muscle cell
- PBS
phosphate-buffered saline
- PDE
phosphodiesterase
- PIP2
phosphatidylinositol 4,5-bisphosphate
- PKA
protein kinase A
- PKC
protein kinase C
- PLA
proximity ligation assay
- VSMC
vascular smooth muscle cell
Authorship Contributions
Participated in research design: Mani, Brueggemann, Cribbs, Byron.
Conducted experiments: Mani, Robakowski, Brueggemann, Tripathi.
Contributed new reagents or analytic tools: Cribbs.
Performed data analysis: Mani, Robakowski, Brueggemann, Tripathi.
Wrote or contributed to the writing of the manuscript: Mani, Robakowski, Brueggemann, Tripathi, Majetschak, Byron.
Footnotes
This work was supported by a grant from the National Institutes of Health National Heart, Lung, and Blood Institute [Grant R01-HL089564] to K.L.B., and predoctoral fellowships from the American Heart Association [09PRE2260209] and Arthur J. Schmitt Foundation to B.K.M.
References
- Ahmad F, Murata T, Shimizu K, Degerman E, Maurice D, Manganiello V. (2015) Cyclic nucleotide phosphodiesterases: important signaling modulators and therapeutic targets. Oral Dis 21:e25–e50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barhanin J, Lesage F, Guillemare E, Fink M, Lazdunski M, Romey G. (1996) KVLQT1 and lsK (minK) proteins associate to form the IKS cardiac potassium current. Nature 384:78–80. [DOI] [PubMed] [Google Scholar]
- Brown DA. (2008) Kv7 (KCNQ) potassium channels that are mutated in human diseases. J Physiol 586:1781–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brueggemann LI, Haick JM, Cribbs LL, Byron KL. (2014a) Differential activation of vascular smooth muscle Kv7.4, Kv7.5, and Kv7.4/7.5 channels by ML213 and ICA-069673. Mol Pharmacol 86:330–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brueggemann LI, Haick JM, Neuburg S, Tate S, Randhawa D, Cribbs LL, Byron KL. (2014b) KCNQ (Kv7) potassium channel activators as bronchodilators: combination with a β2-adrenergic agonist enhances relaxation of rat airways. Am J Physiol Lung Cell Mol Physiol 306:L476–L486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brueggemann LI, Kakad PP, Love RB, Solway J, Dowell ML, Cribbs LL, Byron KL. (2012) Kv7 potassium channels in airway smooth muscle cells: signal transduction intermediates and pharmacological targets for bronchodilator therapy. Am J Physiol Lung Cell Mol Physiol 302:L120–L132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brueggemann LI, Mackie AR, Cribbs LL, Freda J, Tripathi A, Majetschak M, Byron KL. (2014c) Differential protein kinase C-dependent modulation of Kv7.4 and Kv7.5 subunits of vascular Kv7 channels. J Biol Chem 289:2099–2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brueggemann LI, Mackie AR, Martin JL, Cribbs LL, Byron KL. (2011) Diclofenac distinguishes among homomeric and heteromeric potassium channels composed of KCNQ4 and KCNQ5 subunits. Mol Pharmacol 79:10–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brueggemann LI, Moran CJ, Barakat JA, Yeh JZ, Cribbs LL, Byron KL. (2007) Vasopressin stimulates action potential firing by protein kinase C-dependent inhibition of KCNQ5 in A7r5 rat aortic smooth muscle cells. Am J Physiol Heart Circ Physiol 292:H1352–H1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byron KL, Taylor CW. (1993) Spontaneous Ca2+ spiking in a vascular smooth muscle cell line is independent of the release of intracellular Ca2+ stores. J Biol Chem 268:6945–6952. [PubMed] [Google Scholar]
- Chadha PS, Jepps TA, Carr G, Stott JB, Zhu HL, Cole WC, Greenwood IA. (2014) Contribution of kv7.4/kv7.5 heteromers to intrinsic and calcitonin gene-related peptide-induced cerebral reactivity. Arterioscler Thromb Vasc Biol 34:887–893. [DOI] [PubMed] [Google Scholar]
- Chadha PS, Zunke F, Zhu HL, Davis AJ, Jepps TA, Olesen SP, Cole WC, Moffatt JD, Greenwood IA. (2012) Reduced KCNQ4-encoded voltage-dependent potassium channel activity underlies impaired β-adrenoceptor-mediated relaxation of renal arteries in hypertension. Hypertension 59:877–884. [DOI] [PubMed] [Google Scholar]
- Chambard JM, Ashmore JF. (2005) Regulation of the voltage-gated potassium channel KCNQ4 in the auditory pathway. Pflugers Arch 450:34–44. [DOI] [PubMed] [Google Scholar]
- Chen L, Kass RS. (2011) A-kinase anchoring protein 9 and IKs channel regulation. J Cardiovasc Pharmacol 58:459–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuíñas A, Elíes J, Orallo F, Campos-Toimil M. (2013) Cyclic AMP relaxation of rat aortic smooth muscle is mediated in part by decrease of depletion of intracellular Ca2+ stores and inhibition of capacitative calcium entry. Vascul Pharmacol 58:98–104. [DOI] [PubMed] [Google Scholar]
- Delmas P, Brown DA. (2005) Pathways modulating neural KCNQ/M (Kv7) potassium channels. Nat Rev Neurosci 6:850–862. [DOI] [PubMed] [Google Scholar]
- Dunkerley HA, Tilley DG, Palmer D, Liu H, Jimmo SL, Maurice DH. (2002) Reduced phosphodiesterase 3 activity and phosphodiesterase 3A level in synthetic vascular smooth muscle cells: implications for use of phosphodiesterase 3 inhibitors in cardiovascular tissues. Mol Pharmacol 61:1033–1040. [DOI] [PubMed] [Google Scholar]
- Henderson KK, Byron KL. (2007) Vasopressin-induced vasoconstriction: two concentration-dependent signaling pathways. J Appl Physiol (1985) 102:1402–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata Y, Tomita M, Ikeda M. (1985) Characterization of alpha- and beta-adrenergic and angiotensin receptors in cultured vascular smooth muscle cells of rat aorta. Jpn Circ J 49:1043–1051. [DOI] [PubMed] [Google Scholar]
- Jentsch TJ. (2000) Neuronal KCNQ potassium channels: physiology and role in disease. Nat Rev Neurosci 1:21–30. [DOI] [PubMed] [Google Scholar]
- Jepps TA, Greenwood IA, Moffatt JD, Sanders KM, Ohya S. (2009) Molecular and functional characterization of Kv7 K+ channel in murine gastrointestinal smooth muscles. Am J Physiol Gastrointest Liver Physiol 297:G107–G115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi S, Sedivy V, Hodyc D, Herget J, Gurney AM. (2009) KCNQ modulators reveal a key role for KCNQ potassium channels in regulating the tone of rat pulmonary artery smooth muscle. J Pharmacol Exp Ther 329:368–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanamiri S, Soltysinska E, Jepps TA, Bentzen BH, Chadha PS, Schmitt N, Greenwood IA, Olesen SP. (2013) Contribution of Kv7 channels to basal coronary flow and active response to ischemia. Hypertension 62:1090–1097. [DOI] [PubMed] [Google Scholar]
- Kimes BW, Brandt BL. (1976) Characterization of two putative smooth muscle cell lines from rat thoracic aorta. Exp Cell Res 98:349–366. [DOI] [PubMed] [Google Scholar]
- Kubisch C, Schroeder BC, Friedrich T, Lütjohann B, El-Amraoui A, Marlin S, Petit C, Jentsch TJ. (1999) KCNQ4, a novel potassium channel expressed in sensory outer hair cells, is mutated in dominant deafness. Cell 96:437–446. [DOI] [PubMed] [Google Scholar]
- Lee S, Yang Y, Tanner MA, Li M, Hill MA. (2015) Heterogeneity in Kv7 channel function in the cerebral and coronary circulation. Microcirculation 22:109–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerche C, Scherer CR, Seebohm G, Derst C, Wei AD, Busch AE, Steinmeyer K. (2000) Molecular cloning and functional expression of KCNQ5, a potassium channel subunit that may contribute to neuronal M-current diversity. J Biol Chem 275:22395–22400. [DOI] [PubMed] [Google Scholar]
- Li Y, Gamper N, Hilgemann DW, Shapiro MS. (2005) Regulation of Kv7 (KCNQ) K+ channel open probability by phosphatidylinositol 4,5-bisphosphate. J Neurosci 25:9825–9835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes CM, Remon JI, Matavel A, Sui JL, Keselman I, Medei E, Shen Y, Rosenhouse-Dantsker A, Rohacs T, Logothetis DE. (2007) Protein kinase A modulates PLC-dependent regulation and PIP2-sensitivity of K+ channels. Channels (Austin) 1:124–134. [DOI] [PubMed] [Google Scholar]
- Mackie AR, Brueggemann LI, Henderson KK, Shiels AJ, Cribbs LL, Scrogin KE, Byron KL. (2008) Vascular KCNQ potassium channels as novel targets for the control of mesenteric artery constriction by vasopressin, based on studies in single cells, pressurized arteries, and in vivo measurements of mesenteric vascular resistance. J Pharmacol Exp Ther 325:475–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani BK, Brueggemann LI, Cribbs LL, Byron KL. (2009) Opposite regulation of KCNQ5 and TRPC6 channels contributes to vasopressin-stimulated calcium spiking responses in A7r5 vascular smooth muscle cells. Cell Calcium 45:400–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani BK, Brueggemann LI, Cribbs LL, Byron KL. (2011) Activation of vascular KCNQ (Kv7) potassium channels reverses spasmogen-induced constrictor responses in rat basilar artery. Br J Pharmacol 164:237–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani BK, O’Dowd J, Kumar L, Brueggemann LI, Ross M, Byron KL. (2013) Vascular KCNQ (Kv7) potassium channels as common signaling intermediates and therapeutic targets in cerebral vasospasm. J Cardiovasc Pharmacol 61:51–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurice DH, Palmer D, Tilley DG, Dunkerley HA, Netherton SJ, Raymond DR, Elbatarny HS, Jimmo SL. (2003) Cyclic nucleotide phosphodiesterase activity, expression, and targeting in cells of the cardiovascular system. Mol Pharmacol 64:533–546. [DOI] [PubMed] [Google Scholar]
- McCallum LA, Greenwood IA, Tribe RM. (2009) Expression and function of Kv7 channels in murine myometrium throughout oestrous cycle. Pflugers Arch 457:1111–1120. [DOI] [PubMed] [Google Scholar]
- Morales-Cano D, Moreno L, Barreira B, Pandolfi R, Chamorro V, Jimenez R, Villamor E, Duarte J, Perez-Vizcaino F, Cogolludo A. (2015) Kv7 channels critically determine coronary artery reactivity: left–right differences and down-regulation by hyperglycaemia. Cardiovasc Res 106:98–108. [DOI] [PubMed] [Google Scholar]
- Morgado M, Cairrão E, Santos-Silva AJ, Verde I. (2012) Cyclic nucleotide-dependent relaxation pathways in vascular smooth muscle. Cell Mol Life Sci 69:247–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng FL, Davis AJ, Jepps TA, Harhun MI, Yeung SY, Wan A, Reddy M, Melville D, Nardi A, Khong TK, et al. (2011) Expression and function of the K+ channel KCNQ genes in human arteries. Br J Pharmacol 162:42–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obenauer JC, Cantley LC, Yaffe MB. (2003) Scansite 2.0: Proteome-wide prediction of cell signaling interactions using short sequence motifs. Nucleic Acids Res 31:3635–3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohya S, Asakura K, Muraki K, Watanabe M, Imaizumi Y. (2002) Molecular and functional characterization of ERG, KCNQ, and KCNE subtypes in rat stomach smooth muscle. Am J Physiol Gastrointest Liver Physiol 282:G277–G287. [DOI] [PubMed] [Google Scholar]
- Oliveras A, Roura-Ferrer M, Solé L, de la Cruz A, Prieto A, Etxebarria A, Manils J, Morales-Cano D, Condom E, Soler C, et al. (2014) Functional assembly of Kv7.1/Kv7.5 channels with emerging properties on vascular muscle physiology. Arterioscler Thromb Vasc Biol 34:1522–1530. [DOI] [PubMed] [Google Scholar]
- Pattnaik BR, Hughes BA. (2012) Effects of KCNQ channel modulators on the M-type potassium current in primate retinal pigment epithelium. Am J Physiol Cell Physiol 302:C821–C833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polson JB, Strada SJ. (1996) Cyclic nucleotide phosphodiesterases and vascular smooth muscle. Annu Rev Pharmacol Toxicol 36:403–427. [DOI] [PubMed] [Google Scholar]
- Robbins J. (2001) KCNQ potassium channels: physiology, pathophysiology, and pharmacology. Pharmacol Ther 90:1–19. [DOI] [PubMed] [Google Scholar]
- Schroeder BC, Hechenberger M, Weinreich F, Kubisch C, Jentsch TJ. (2000) KCNQ5, a novel potassium channel broadly expressed in brain, mediates M-type currents. J Biol Chem 275:24089–24095. [DOI] [PubMed] [Google Scholar]
- Schroeder BC, Kubisch C, Stein V, Jentsch TJ. (1998) Moderate loss of function of cyclic-AMP-modulated KCNQ2/KCNQ3 K+ channels causes epilepsy. Nature 396:687–690. [DOI] [PubMed] [Google Scholar]
- Schwake M, Jentsch TJ, Friedrich T. (2003) A carboxy-terminal domain determines the subunit specificity of KCNQ K+ channel assembly. EMBO Rep 4:76–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedivy V, Joshi S, Ghaly Y, Mizera R, Zaloudikova M, Brennan S, Novotna J, Herget J, Gurney AM. (2015) Role of Kv7 channels in responses of the pulmonary circulation to hypoxia. Am J Physiol Lung Cell Mol Physiol 308:L48–L57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims SM, Singer JJ, Walsh JV., Jr (1985) Cholinergic agonists suppress a potassium current in freshly dissociated smooth muscle cells of the toad. J Physiol 367:503–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims SM, Singer JJ, Walsh JV., Jr (1988) Antagonistic adrenergic-muscarinic regulation of M current in smooth muscle cells. Science 239:190–193. [DOI] [PubMed] [Google Scholar]
- Söderberg O, Leuchowius KJ, Gullberg M, Jarvius M, Weibrecht I, Larsson LG, Landegren U. (2008) Characterizing proteins and their interactions in cells and tissues using the in situ proximity ligation assay. Methods 45:227–232. [DOI] [PubMed] [Google Scholar]
- Suh BC, Hille B. (2007) Regulation of KCNQ channels by manipulation of phosphoinositides. J Physiol 582:911–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svalø J, Bille M, Parameswaran Theepakaran N, Sheykhzade M, Nordling J, Bouchelouche P. (2013) Bladder contractility is modulated by Kv7 channels in pig detrusor. Eur J Pharmacol 715:312–320. [DOI] [PubMed] [Google Scholar]
- Tripathi A, Vana PG, Chavan TS, Brueggemann LI, Byron KL, Tarasova NI, Volkman BF, Gaponenko V, Majetschak M. (2015) Heteromerization of chemokine (C-X-C motif) receptor 4 with α1A/B-adrenergic receptors controls α1-adrenergic receptor function. Proc Natl Acad Sci USA 112:E1659–E1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HS, Pan Z, Shi W, Brown BS, Wymore RS, Cohen IS, Dixon JE, McKinnon D. (1998) KCNQ2 and KCNQ3 potassium channel subunits: molecular correlates of the M-channel. Science 282:1890–1893. [DOI] [PubMed] [Google Scholar]
- Wells JN, Kramer GL. (1981) Phosphodiesterase inhibitors as tools in cyclic nucleotide research: a precautionary comment. Mol Cell Endocrinol 23:1–9. [DOI] [PubMed] [Google Scholar]
- Wickenden AD. (2002) Potassium channels as anti-epileptic drug targets. Neuropharmacology 43:1055–1060. [DOI] [PubMed] [Google Scholar]
- Yang CM, Chiu CT, Wang CC, Tsao HL, Fan LW. (1999) Forskolin inhibits 5-hydroxytryptamine-induced phosphoinositide hydrolysis and Ca2+ Mobilisation in canine cultured aorta smooth muscle cells. Cell Signal 11:697–704. [DOI] [PubMed] [Google Scholar]
- Yeung SY, Pucovský V, Moffatt JD, Saldanha L, Schwake M, Ohya S, Greenwood IA. (2007) Molecular expression and pharmacological identification of a role for Kv7 channels in murine vascular reactivity. Br J Pharmacol 151:758–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Wu M, Townsend SD, Zou B, Long S, Daniels JS, McManus OB, Li M, Lindsley CW, Hopkins CR. (2011) Discovery, synthesis, and structure–activity relationship of a series of N-aryl-bicyclo[2.2.1]heptane-2-carboxamides: characterization of ML213 as a novel KCNQ2 and KCNQ4 potassium channel opener. ACS Chem Neurosci 2:572–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaydman MA, Cui J. (2014) PIP2 regulation of KCNQ channels: biophysical and molecular mechanisms for lipid modulation of voltage-dependent gating. Front Physiol 5:195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao YJ, Wang J, Rubin LJ, Yuan XJ. (1998) Roles of K+ and Cl− channels in cAMP-induced pulmonary vasodilation. Exp Lung Res 24:71–83. [DOI] [PubMed] [Google Scholar]
- Zhong XZ, Harhun MI, Olesen SP, Ohya S, Moffatt JD, Cole WC, Greenwood IA. (2010) Participation of KCNQ (Kv7) potassium channels in myogenic control of cerebral arterial diameter. J Physiol 588:3277–3293. [DOI] [PMC free article] [PubMed] [Google Scholar]