Figure 5.
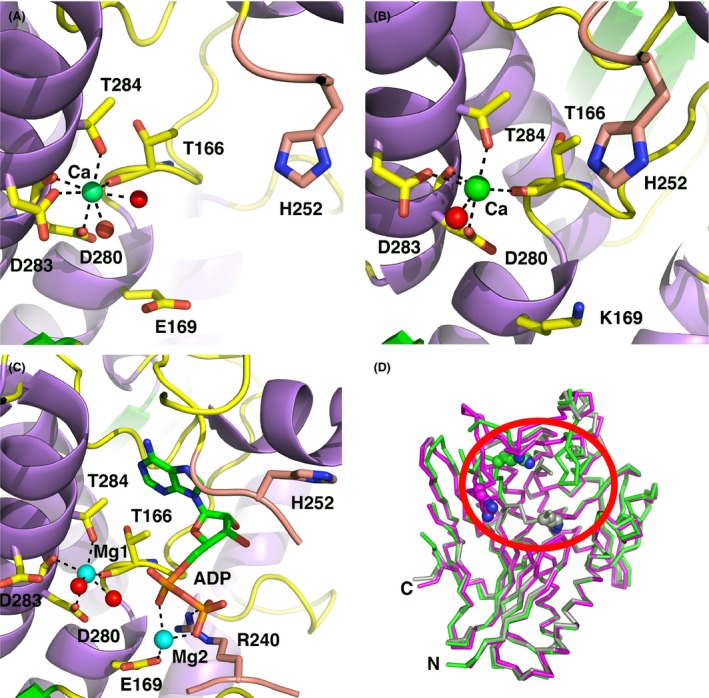
Active‐site geometries of Ftp_Ec structures. The carbon atoms of protein side chains are yellow, the carbon atoms from residues of the β‐hairpin insert are salmon, the nucleotides atoms are green, Ca2+ ions are green spheres, Mg2+ ions are cyan spheres, waters are red spheres, nitrogens are blue, and oxygens are red. Black dotted lines represent metal first‐coordination‐sphere contacts and important hydrogen bonding interactions. For clarity, some protein residues have been selectively removed from the images. (A) Apo wild‐type Ftp_Ec, monomer A. (B) Apo Ftp_EcE169K, monomer A. (C) Ftp_EcY60N Mg2+‐ADP complex, monomer A. (D) Cα superposition of three representative Ftp_Ec monomer structures. Circled in red is the active site loop region (residues 247‐253) that displays variable conformations amongst the structures. Monomer A of Ftp_Ec wild‐type is colored green, monomer B of Ftp_Ec wild‐type is colored magenta, and monomer A of Ftp_EcY60N is colored gray. Shown as a cyan sphere is the Ca2+ ion bound to metal site 1. Shown as color‐coded spheres is R240 in all three structures.
