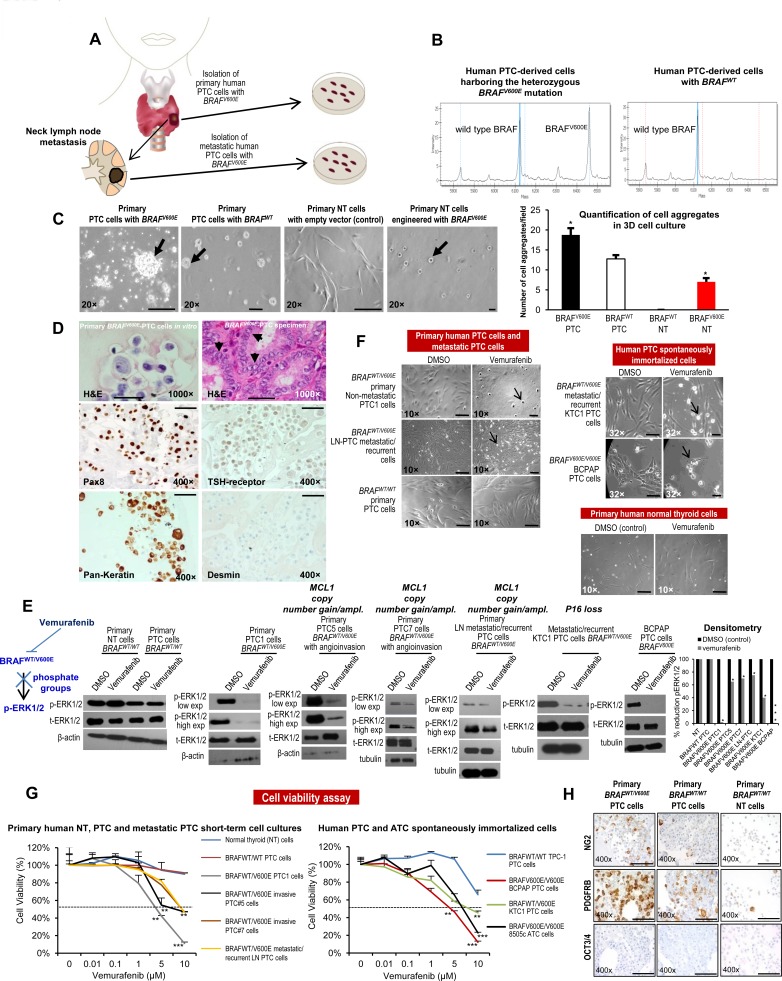
Figure 1. In vitro preclinical model of human papillary thyroid cancer (PTC) harboring the BRAFV600E mutation.
A. Experimental design of an in vitro and in vivo model of human PTC with the BRAFWT/V600E mutation. B. DNA genotyping analysis of human PTC identifies the heterozygous BRAFWT/V600E mutation. Mass spectrometry (MS) traces of human primary PTC cells. The intensity of the signal versus mass of the analyte is plotted in the background. Calls are based on an expected allelic frequency of 50%. Allele frequencies deviating from the expected values are assigned ambiguous or homozygous calls by the software. MS trace of PTC cells reveals a heterozygous BRAFWT/V600E allele (A>T). C. In a three dimensional (3D) cell culture assay using reconstituted basement membrane extracellular matrix (ECM) (Matrigel), BRAFV600E-PTC cells grew as larger cell aggregates. Normal thyroid (NT) cells transduced with BRAFV600E grew as adherent refractile cells vs. NT cells engineered with empty vector (control) which grew as spindled cells. Scale bar= 400 μ, 200 μ, 400 μ and 50 μ, respectively. D. Immunocytochemistry of representative established short-term primary human PTC cells in vitro with the heterozygous BRAFWT/V600E mutation of patient-PTC specimen (Hematoxylin-Eosin, H&E, arrows highlight nuclear clearing). Immunocytochemistry staining in the PTC cells in vitro shows cytoplasmic to membranous staining with antibodies against PAX8, TSH-receptor, and pan-keratin (marker of tumor epithelial cells and tumor purity). Desmin immunostain was negative. Scale bars= 500 μ (1000× magnification image) and 100 μ (400× magnification images). E. Inhibition of BRAFWT/V600E by vemurafenib reduces phospho(p)ERK1/2 protein expression levels. A parallel plate similar to F was set up and corresponding pERK1/2 protein levels (low exp= shorter exposure during chemiluminescence reaction; high exp= longer exposure during chemiluminescence reaction) were measured from BRAFWT/V600E-PTC cells, BRAFWT-PTC cells, or NT cells by western blotting. Densitometry analysis of the pERK1/2 protein levels in NT or PTC cells treated with 10 μM vemurafenib vs. vehicle (DMSO =Dimethyl sulfoxide, control) for 24 hours, in the corresponding western blotting (*p < 0.05, Mann-Whitney test). Primary BRAFWT-NT cells have MCL1 neutral copy number, primary BRAFWT-PTC cells have MCL1 copy number =0.9, primary non-metastatic BRAFWT/V600E-PTC1 cells have MCL1 copy number =2.14, primary BRAFWT/V600E-PTC5 cells with angio-invasion have MCL1 copy number =3, primary BRAFWT/V600E-PTC7 cells with angio-invasion have MCL1 copy number =3, primary LN metastatic/recurrent BRAFV600E-PTC cells have MCL1 copy number =3.8, KTC1 cells have MCL1 copy number =1.3 and BCPAP cells have MCL1 copy number =1.4. KTC1 cells have P16 homozygous loss. For more details regarding copy number gain/amplification (ampl.) assay see Figure 4 and Methods. These data are representative of three independent experiments. We show these results in the 5 out of 7 short-term primary human PTC cell cultures which grew well. F. Arrows highlight change of cell shape in BRAFWT/V600E-PTC cells treated with vemurafenib vs. vehicle-treated (control) PTC cells. PTC cells with heterozygous BRAFWT/V600E or BRAFWT or NT cells were treated with 10 μM of vemurafenib or with DMSO (control) for about 24 hours. These data represent 3 independent experiments. All scale bars are=50 μ (DMSO images) and 10 μ (Vemurafenib images). Scale bars are =50 μ (BRAFWT/WT primary PTC cells and primary human normal thyroid cells images). G. Vemurafenib dose-reponse analysis: short-term primary human PTC or NT cells with BRAFV600E or with BRAFWT, as well as spontaneously immortalized human PTC and ATC cells, were treated with the indicated concentrations of vemurafenib for 48 hours, and viability was determined using the Cell Titer-Glo ATP-based luminescence assay, with DMSO-treated cells as the control. We show these results in the 5 out of 7 short-term primary human PTC cell cultures which grew well. These data represent the average ± standard deviation (error bars) of 3-5 independent replicate measurements (*p < 0.05, **p < 0.01, ***p < 0.001, Mann-Whitney test). H. Immunocytochemistry of representative established non-immortalized primary human PTC cells with the heterozygous BRAFWT/V600E mutation or with BRAFWT, or NT cells. Immunohistochemistry staining shows cytoplasmic to membranous staining with antibodies against NG2 or PDGFRB (platelet-derived growth factor receptor-beta) in BRAFWT/V600E-PTC or BRAFWT-PTC cells. OCT3/4 immunostain was negative. Markers expression was assessed semiquantitatively using the following scoring method: 0 (negative), 1+ (1–10% positive cells), 2+ (11–50% positive cells), and 3+ (more than 50% positive cells). All scale bars are=100 μ.