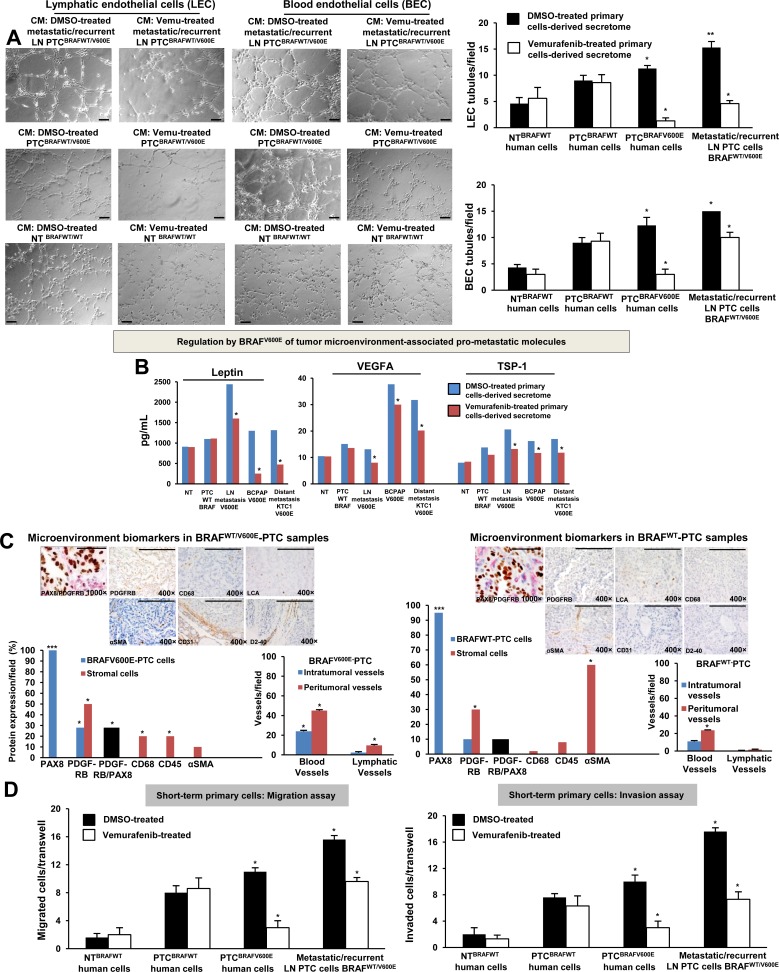
Figure 3. BRAFV600E-positive papillary thyroid carcinoma (PTC) cells promote in vitro angiogenesis (tubule-like structures formation) using patient-derived preclinical models.
A. In vitro evaluation of microvascular endothelial cell tube formation. Secretome from representative short-term primary human heterozygous BRAFWT/V600E-positive PTC triggered human lymphatic and blood microvascular endothelial cell (LEC and BEC, respectively) tube formation in vitro. LEC and BEC were suspended in secretome (conditioning medium (CM)) derived from short-term primary NT or PTC cells with BRAFWT/V600E or BRAFWTtreated with vehicle (DMSO) or vemurafenib (10 μM) for 24 hours and seeded on growth factor–reduced Matrigel. The secretome was utilized to induce tube formation. LEC and BEC tubes were photographed after 5-6 hours. Histograms show numbers of LEC and BEC tubes triggered by non-metastatic BRAFWT/V600E PTC-derived secretome, metastatic/recurrent BRAFWT/V600E PTC-derived secretome, or in the presence of secretome derived from primary NT cells or PTC cells with BRAFWT. Magnification: 10×. All scale bars are=200 μ. These data represent the average ± standard deviation (error bars) of 2 independent experiments replicate measurements (*p < 0.05, **p < 0.01, ***p < 0.001, Mann-Whitney test). B. Measurements of Leptin, VEGFA and TSP-1 secreted protein levels in the vehicle-treated or vemurafenib-treated secretome derived from representative: short-term primary NT cells, short-term primary metastatic/recurrent BRAFWT/V600E-PTC cells, spontaneously immortalized human PTC-derived BCPAP cells (homozygous BRAFV600E) and metastatic PTC-derived spontaneously immortalized human KTC1 cells (heterozygous BRAFV600E). NT or PTC cells were cultured in vitro in the presence of vemurafenib (10 μM) or vehicle (DMSO) for 24 hours. The secretome (growth medium enriched with PTC or NT-derived growth/angiogenic factors) was collected and protein levels (pg/mL) were determined by ELISA (enzyme-linked immunosorbent assay). Protein levels were normalized to total protein content (μg/μL). These data represent the average ± standard deviation (error bars) of 2 independent experiments replicate measurements (*p < 0.05, Mann-Whitney test). C. Assessment of PTC microenvironment in formalin-fixed paraffin-embedded clinical human PTC specimens harboring BRAFWT/V600E (n=4) versus BRAFWT (n=3). Immunohistochemical protein expression of: PAX8 (marker of PTC cells) showed nuclear localization; PDGFRB (platelet-derived growth factor receptor beta, pericyte lineage marker and tumor cell marker) showed cytoplasmic/plasma membrane localization; CD68 (CD68, macrophage marker), localized to plasma membrane; CD45 (also called LCA, leucocyte lineage marker) localized to plasma membrane; and αSMA (alpha Smooth Muscle Actin, markers of smooth muscle cell lineage) mainly localized to plasma membrane. Black bars indicate percent of colocalization between PDGFRB and PAX8. Immunohistochemical protein expression per field was assessed semi-quantitatively using the following scoring method: 0 (negative), 1+ (1–10% positive cells), 2+ (11–50% positive cells), and 3+ (more than 50% positive cells). Microvascular density is defined by number of vessels per microscope field showing CD31 (BEC marker) or D2-40 (Podoplanin, LEC marker) staining. Scale bars are=500 μ (1000× magnification image) and 400 μ (400× magnification images). These data represent the average ± standard deviation (error bars) of independent replicate measurements (*p<0.05, Mann-Whitney test). D. Migration and invasion assays with vemurafenib or DMSO (vehicle, control) treatment in representative short-term primary human PTC cell cultures with heterozygous BRAFWT/V600E or BRAFWT, and in short-term primary human NT cell cultures. Treatment with vemurafenib at 10 μM was performed in the migration and invasion assays. These data represent the average ± standard deviation (error bars) of 2 or 3 independent experiments replicate measurements (*p < 0.05, Mann-Whitney test).