ABSTRACT
A rapid and reliable test for detection of complicated appendicitis would be useful when deciding whether emergency surgery is required. We investigated the clinical usefulness of procalcitonin for identifying acute complicated appendicitis. We retrospectively analyzed 63 patients aged ≥15 years who underwent appendectomy without receiving antibiotics before admission and had preoperative data on the plasma procalcitonin level (PCT), body temperature (BT), white blood cell count (WBC), neutrophil / lymphocyte ratio (N/L ratio), and C-reactive protein level (CRP). Patients were classified into 3 groups: group A (inflammatory cell infiltration of the appendix with intact mural architecture), group B (inflammatory cell infiltration with destruction of mural architecture, but without abscess or perforation), and group C (macroscopic abscess and/or perforation). For identifying destruction of mural architecture, the diagnostic accuracy of PCT was similar to that of BT or CRP. However, the diagnostic accuracy of PCT was highest among the five inflammatory indices for identifying abscess and/or perforation, with the positive predictive value of PCT for abscess and/or perforation being higher than that of CRP (73% vs. 48%). Univariate analysis of the predictors of abscess and/or perforation revealed that a plasma PCT level ≥0.46 ng/mL had the highest odds ratio (30.3 [95% confidence interval: 6.5–140.5] versus PCT <0.46 ng/mL). These findings indicate that procalcitonin is a useful marker of acute appendicitis with abscess and/or perforation.
Key Words: procalcitonin, appendicitis, C-reactive protein
INTRODUCTION
Acute appendicitis is the most common abdominal surgical emergency. While recent advances in computed tomography and ultrasound have improved the accuracy of diagnosis, it is still often difficult to differentiate between simple and complicated appendicitis, which is usually defined as appendicitis with gangrenous change, abscess, or perforation.1-3) Conservative management with antibiotics and supportive therapy is an option for patients with early uncomplicated appendicitis, and the condition resolves in most cases.4-8) A recent meta-analysis of randomized controlled trials confirmed that initial treatment of early uncomplicated appendicitis with antibiotics merits consideration.9) However, a large group of patients present with complicated appendicitis and delay in making the correct diagnosis and initiating surgical treatment may lead to ruptured appendix with generalized sepsis. Therefore, a rapid and reliable test that can detect or exclude gangrenous change, abscess, or perforation of the appendix would be useful when deciding whether emergency surgery is required.
Some widely available laboratory tests can provide assistance in determining the severity of acute appendicitis. Conventional clinical or laboratory parameters, including the body temperature (BT), white blood cell count (WBC), neutrophil / lymphocyte ratio (N/L ratio), and C-reactive protein (CRP) level, have been employed to distinguish between uncomplicated and complicated appendicitis.10,11) In 1993, Assicot et al. reported a marked increase of the plasma procalcitonin (PCT) level in patients with sepsis and other clinically significant bacterial infections.12) PCT is a precursor of calcitonin. It is constitutively secreted by the C cells of the thyroid gland and the K cells of the lungs, and is also rapidly produced by most parenchymal tissues throughout the body in response to stimulation by endotoxin or inflammatory cytokines.13-15) Unlike CRP, the PCT level does not increase in patients with sterile inflammation or viral infection.12) This characteristic makes it a popular biomarker for many conditions, including acute appendicitis. Recent studies have demonstrated that PCT is useful as a marker for detection of sepsis and for estimating the severity of pneumonia, pancreatitis, or bacteremia.16-20) Several previous studies have investigated the value of PCT for diagnosing or determining the severity of acute appendicitis, but the results have been inconsistent.21-26) Accordingly, we investigated the usefulness of PCT as a diagnostic marker for complicated appendicitis compared with BT, WBC, the N/L ratio, and CRP.
PATIENTS AND METHODS
We retrospectively analyzed the medical records of 63 patients aged ≥15 years who underwent appendectomy from November 2011 to October 2013 and had histopathological findings consistent with acute appendicitis. The preoperative clinical diagnosis was acute appendicitis in all patients. For each patient, the age, sex, BT, WBC, N/L ratio, CRP, plasma PCT level, pathological findings, and length of hospital stay were investigated. Patients who had received antibiotics before admission were excluded from this study, because antibiotic therapy can alter laboratory data and BT, and can modify the pathological features of appendicitis.
The plasma PCT level was measured with a Cobas e411 electrochemiluminescence immunoassay (Roche Diagnostics Japan, Tokyo, Japan). Its lower limit of detection was 0.02 ng/mL, and its reportable range (analytical range and clinical reportable range) was between 0.02 and 100 ng/mL. According to the manufacturer, the mean within-run reproducibility at approximately 0.5 ng/mL was 0.55 ng/mL (coefficient of variation: 1.1%). A Coulter LH750 counter (Beckman Coulter, Tokyo, Japan) was used to determine the WBC and the N/L ratio. A JCA-BM2250 analyzer (Japan Electron Optics, Tokyo) and N-assay LA CRP-S D-type analytical reagent (Nittobo Medical, Tokyo, Japan) were used to measure serum CRP. All assays were performed at a single laboratory.
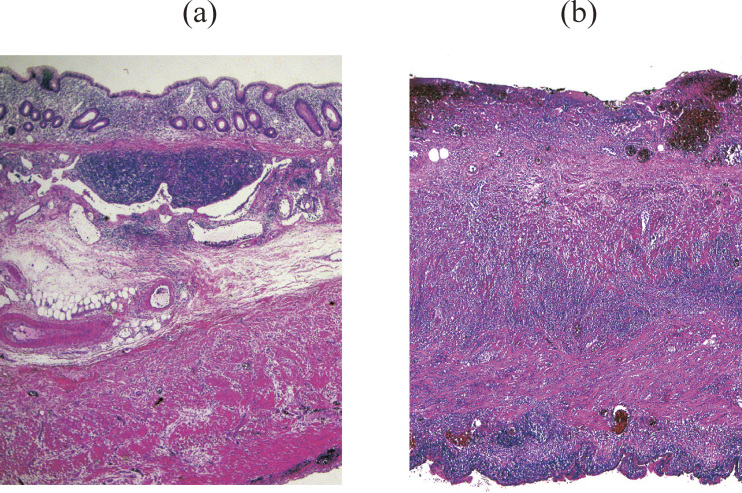
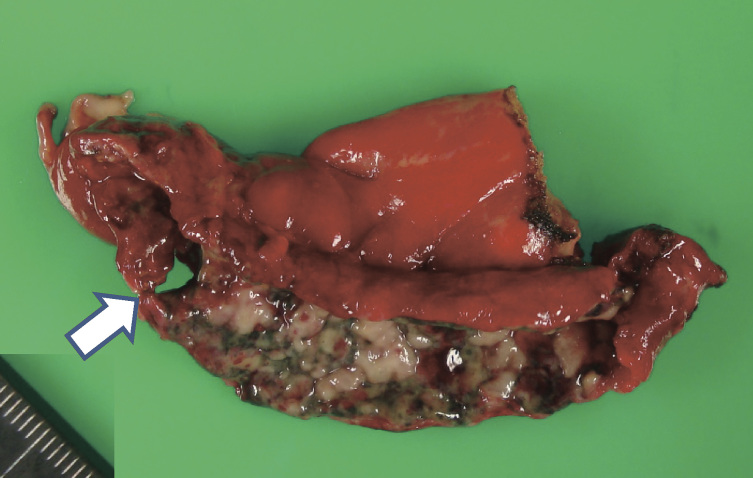
The pathological diagnosis of acute appendicitis was based on intraoperative findings combined with macroscopic and histological examination of the resected appendix. Patients were classified into the following three groups according to the severity of inflammation of the appendix: (1) group A had inflammatory cell infiltration of the appendix with intact mural architecture (Figure 1a); (2) group B had inflammatory cell infiltration with destruction of mural architecture, but without abscess or perforation (Figure 1b), and (3) group C had inflammatory cell infiltration with destruction of mural architecture and a macroscopic peri-appendiceal abscess and/or macroscopic perforation (Figure 2).
Fig. 1.
Microscopic findings of resected appendixes. Inflammatory cell invasion without destruction of the mural architecture (a) and with destruction of the mural architecture (b).
Fig. 2.
Macroscopic appearance of a resected appendix with perforation (arrow).
Outcome Measures
Histopathological destruction of the mural architecture of the appendix is considered to represent gangrenous change, suggesting that conservative therapy with antibiotics is not indicated, while an abscess and/or perforation requires immediate surgery. Therefore, the diagnostic accuracy of PCT, BT, WBC, the N/L ratio, and CRP were compared for the following categories of acute appendicitis: (1) appendicitis without vs. with destruction of the mural architecture (group A vs. groups B+C) and (2) appendicitis without vs. with abscess and/or perforation (groups A+B vs. groups C).
Statistical Analysis
Diagnostic accuracy was assessed by calculating the positive predictive value (PPV) and negative predictive value (NPV). Receiver operating characteristic (ROC) analysis was performed and the area under the ROC curve (AUC) was calculated to assess the diagnostic value of PCT, BT, WBC, N/L ratio, and CRP for each category of acute appendicitis. Differences of AUC values were analyzed with the DeLong test.27) The optimal cut-off value was determined as the point of maximum distance between the ROC curve and the diagonal line.
Continuous variables are expressed as the mean ± standard deviation, unless otherwise stated. Logarithmic transformation of plasma PCT data was performed for graphic display. Nonparametric comparisons between two groups were done with the Mann-Whitney U test. For comparison among multiple groups, Kruskal-Wallis nonparametric analysis of variance was performed, while the Mann-Whitney U test with Bonferroni’s correction was used for post hoc comparisons. All tests were two-tailed. A probability (P) <0.05 was considered to indicate statistical significance, except that P<0.01 was accepted for the Mann-Whitney U test with Bonferroni’s correction. Logistic regression was performed for univariate analysis of the predictors of appendiceal abscess and/or perforation. All statistical analyses were performed with JMP (Version 11; SAS Institute, Japan) or modified R software (The R Foundation for Statistical Computing, Perugia, Italy). Our institutional ethics committee determined that this study did not require ethical approval.
RESULTS
Subjects
A total of 195 patients underwent appendectomy at our department during the study period. After excluding patients who were under 15 years old, those who received antibiotics before admission, those without complete data (PCT, BT, WBC, N/L ratio, and CRP), and those without histopathological evidence of acute appendicitis, 63 patients (32%) were entered into the study. Their mean age was 48 ± 18 years, and 39 patients (62%) were male. The number of patients in groups A, B, and C was 14, 34, and 15, respectively. None of the patients died after appendectomy. The demographic profile and length of hospital stay in groups A, B, and C are presented in Table 1. Groups B and C were older than group A, while the hospital stay was longest in group C among the three groups.
Table 1.
Demographic profile and hospital stay
| Total | Group A | Group B | Group C | P value | ||
|---|---|---|---|---|---|---|
| (n=63) | (n=14) | (n=34) | (n=15) | |||
| Age (years) : median | 46 | 35 | 49 | 49 | 0.0032 | |
| interquartile range | 27 | 14 | 26 | 33 | ||
| Male : Female ratio | 39:24 | 8:6 | 23:11 | 8:7 | 0.5835 | |
| Length of hospital stay (days): median | 5 | 5 | 5 | 12 | <0.0001 | |
| interquartile range | 2.8 | 1 | 1 | 12 | ||
Comparison of Inflammatory Indices
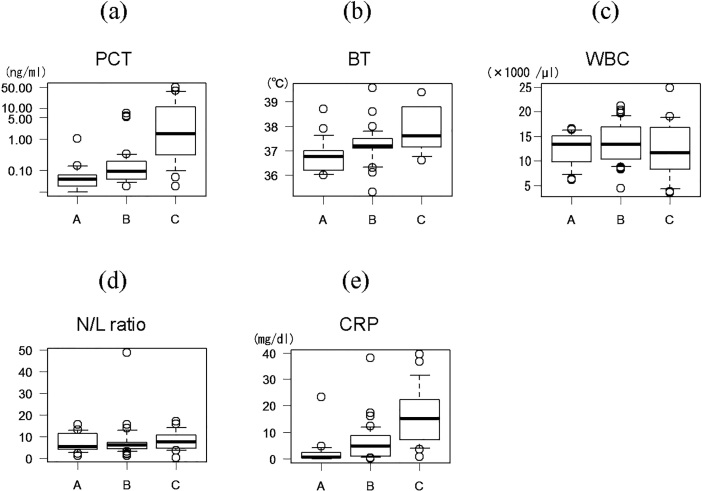
The PCT, BT, WBC, N/L ratio, and CRP of each group are shown in Figure 3. While PCT, BT, and CRP increased across the three groups from A to C, the WBC and N/L ratio did not. PCT, BT, and CRP showed significant differences among the three groups (p<0.0001, p=0.001, and p<0.0001, respectively, Kruskal-Wallis test).
Fig. 3.
Procalcitonin (PCT) (a), body temperature (BT) (b), white blood cell count (WBC) (c), neutrophil/lymphocyte (N/L) ratio (d), and C-reactive protein (CRP) (e) in patients stratified by the macroscopic and histopathological features of the resected appendix. Box and whisker plots: boxes represent the 25th to 75th percentiles, bold horizontal lines indicate the median, and whiskers display the 10th and 90th percentiles.
Diagnostic Value for Complicated Appendicitis
(1) Destruction of mural architecture (group A vs. groups B+C)
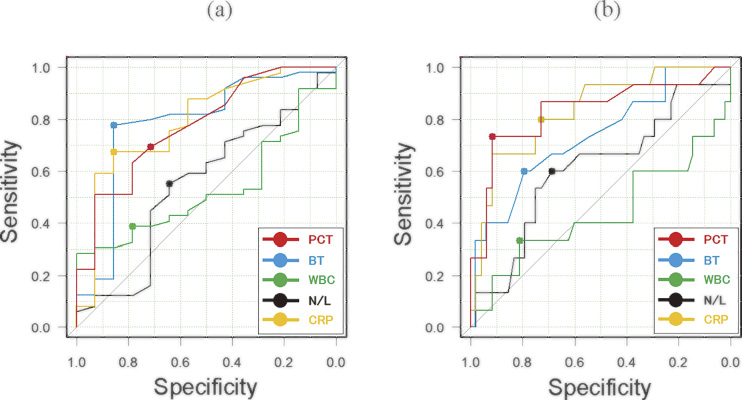
The diagnostic value of PCT, BT, WBC, N/L ratio, and CRP for predicting destruction of the mural architecture (group A vs. groups B+C) is presented in Table 2, while the ROC curves are displayed in Figure 4a. The optimal cut-off values of PCT, BT, WBC, N/L ratio, and CRP were 0.07 ng/mL, 37.1°C, 15.3×103 /μL, 6.2, and 3.5 mg/dL, respectively. The AUCs of PCT, BT, and CRP were similar and the PPV of PCT (90%) was slightly lower than that of BT or CRP.
Table 2.
Diagnostic value of each parameter for destruction of mural structure (group A vs. groups B+C)
| PCT | BT | WBC | N/L ratio | CRP | |
|---|---|---|---|---|---|
| Optimal cutoff value | 0.07 (ng/mL) | 37.1 (°C) | 15.3 (×103/μL) | 6.2 | 3.5 (mg/dL) |
| Positive predictive value | 90 | 95 | 86 | 84 | 94 |
| Negative predictive value | 40 | 52 | 27 | 29 | 43 |
| Area under the ROC curve | 0.777 | 0.781 | 0.531 | 0.527 | 0.792 |
Fig. 4.
Receiver operating characteristic curves for procalcitonin (PCT), body temperature (BT), white blood cell count (WBC), neutrophil/lymphocyte (N/L) ratio, and C-reactive protein (CRP) as predictors of mural destruction (a) and abscess and/or perforation (b).
(2) Abscess and/or perforation (groups A+B vs. group C)
The diagnostic value of PCT, BT, WBC, N/L ratio, and CRP for predicting an appendiceal abscess and/or perforation (groups A+B vs. group C) is presented in Table 3, while the ROC curves are shown in Figure 4b. The optimal cut-off values of PCT, BT, WBC, N/L ratio, and CRP were 0.46 ng/mL, 37.6°C, 16.7×103 μL, 7.3, and 6.7 mg/dL, respectively. The AUC and NPV of PCT were similar to those of CRP, which had the highest values among the five inflammatory indices, but the PPV of PCT (73%) was higher than that of CRP (48%). Univariate analysis of the predictors of abscess and/or perforation revealed that PCT ≥0.46 ng/mL had the highest odds ratio (30.3 [95% confidence interval: 6.5–140.5] versus PCT <0.46 ng/mL) among the five indices investigated (Table 4).
Table 3.
Diagnostic value of each inflammatory parameter for abscess and/or perforation (groups A+B vs. group C)
| PCT | BT | WBC | N/L ratio | CRP | |
|---|---|---|---|---|---|
| Optimal cutoff value | 0.46 (ng/mL) | 37.6 (°C) | 16.7 (×103/μL) | 7.3 | 6.7 (mg/dL) |
| Positive predictive value | 73 | 47 | 36 | 38 | 48 |
| Negative predictive value | 92 | 86 | 80 | 85 | 92 |
| Area under the ROC curve | 0.832 | 0.733 | 0.439 | 0.609 | 0.830 |
Table 4.
Univariate analysis of the predictors of abscess and/or perforation
| Variable | P value | Odds ratio | 95% confidence interval | |
|---|---|---|---|---|
| PCT | <0.46 | 1 (reference) | ||
| ≥0.46 | <0.0001 | 30.3 | 6.5–140.5 | |
| BT | <37.6 | 1 (reference) | ||
| ≥37.6 | 0.0062 | 5.7 | 1.6–19.8 | |
| WBC | <16700 | 1 (reference) | ||
| ≥16700 | 0.242 | 2.2 | 0.6–7.9 | |
| N/L ratio | <7.3 | 1 (reference) | ||
| ≥7.3 | 0.0511 | 3.3 | 1.00–11.0 | |
| CRP | <6.7 | 1 (reference) | ||
| ≥6.7 | 0.001 | 10.8 | 2.6–44.4 |
DISCUSSION
In the present study, we assessed the diagnostic accuracy of five inflammatory indices (PCT, BT, WBC, the N/L ratio, and CRP) for predicting destruction of the mural architecture of the appendix and the presence of abscess and/or perforation. For identifying destruction of the mural architecture, the diagnostic accuracy of PCT was similar to that of BT or CRP. However, the diagnostic accuracy of PCT was highest among the five inflammatory indices for identifying abscess and/or perforation, with the PPV of PCT (73%) being higher than that of CRP (48%).
Acute appendicitis is a common cause of acute abdomen and can require immediate surgery, with delayed diagnosis and surgical treatment resulting in complications or even death. The reported overall mortality rate of appendicitis is only 0.3%, but this rises considerably in patients with perforation or older patients.28) Perforated appendix is associated with higher morbidity and mortality compared to appendicitis without perforation. In one series, the mortality rate of patients over 80 years old with perforated appendicitis was 21%.29)
Varadhan et al. performed a meta-analysis of randomized controlled trials, and concluded that initial antibiotic therapy merits consideration as a primary treatment option for early uncomplicated appendicitis.9) Conservative management of uncomplicated acute appendicitis with antibiotics and supportive treatment has also been advocated by several other authors,4-8) but it is often difficult to distinguish between uncomplicated and complicated appendicitis.
Various laboratory tests have been investigated for making a diagnosis or evaluating the severity of acute appendicitis, including WBC, the N/L ratio, CRP, PCT, interleukin-6, D-dimer, pancreatic stone protein, and lipopolysaccharide-binding protein. For diagnosing acute appendicitis, PCT was reported to be no better than other markers such as CRP or WBC.23-26) However, several reports have suggested that PCT has a higher diagnostic value for complicated appendicitis.22, 24, 26) Kafetzis et al. assessed the diagnostic value of PCT in 212 children with appendicitis, and found that a PCT level >0.5 ng/ml could identify perforation or gangrene with a sensitivity of 73.4% and a specificity of 94.6%.22) Yu et al. performed a systematic review of seven studies on the diagnostic accuracy of PCT, CRP, and WBC, which showed that PCT was more accurate for diagnosing complicated appendicitis, although they did not provide a definition of complicated appendicitis in their report.26) Anielski et al. reported that measurement of interleukin-6 was useful for reducing the false-positive rate for diagnosis of acute appendicitis.30) Tschuor et al. studied pancreatic stone protein as a predictor of acute appendicitis in patients presenting to the emergency department with abdominal pain,31) and Brănescu et al. evaluated lipopolysaccharide-binding protein in 147 patients admitted with acute appendicitis.32) Although the results of these studies were encouraging, both of these tests are unavailable in the routine clinical setting.
The correlation between the findings of the surgeon at operation and the results of pathological examination is sometimes not complete,33-35) and there are various definitions of the stages of appendicitis in the literature.36, 37) In this study, we defined the stages of acute appendicitis based on intraoperative findings (peri-appendiceal abscess), macroscopic findings (perforation of the resected specimen), and microscopic findings (destruction of mural architecture). We found that PCT had a similar diagnostic value for mural destruction to that of BT or CRP, but PCT was superior to the other inflammatory indices for identifying abscess and/or perforation.
PCT is not only elevated in patients with systemic infections, but is also increased by some localized bacterial infections, such as lower respiratory tract infection, meningitis, and pancreatitis.38, 39) Bacterial lipopolysaccharides and the proinflammatory cytokines are the most potent inducers of PCT production.40) Brunkhorst et al. showed that injection of bacterial endotoxin into healthy subjects caused the PCT level to rise by approximately 0.5 ng/ml per hour after a latent period of about 2–3 h, reaching a plateau after 6–12 h.14) Mild acute appendicitis may not be associated with significant bacterial translocation. Aslan et al. reported that only 3 of 18 patients (16.6%) with acute appendicitis showed bacterial translocation to the mesenteric lymph nodes adjacent to the terminal ileum.41) Once bacterial translocation occurs from the appendix to the lymph nodes or the portal vein, PCT production is induced. PCT has a half-life of 24 to 30 hours, while CRP does not increase for 12 to 24 hours after the onset of inflammation and takes 20 to 72 hours to reach a plateau.14) The divergent behavior of these two inflammatory indices may underlie the difference in the diagnostic value of PCT and CRP.
This study had several limitations. First, the number of patients investigated was relatively small. To allow us to properly assess the diagnostic value of the five inflammatory indices, patients who had received antibiotics before admission were excluded because antibiotic therapy might have influenced both the indices and histopathological changes of the appendix. Conservative management of appendicitis with antibiotics and supportive therapy is frequent in Japan, which reduced the number of patients available for our study. Second, our histopathological classification of acute appendicitis was not the most common one. Unfortunately, the definition of the stages of appendicitis varies in the literature,34, 35) but the results of future studies on acute appendicitis can be compared with ours because we clearly defined each stage. Despite these limitations, we consider that our findings can be applied for determining whether emergency surgery is required in patients with acute appendicitis, since PCT is a useful marker of acute appendicitis associated with abscess or perforation.
Conflicts of Interest and Source of Funding: None
REFERENCES
- 1).Martin AE, Vollman D, Adler B, Caniano DA. CT scans may not reduce the negative appendectomy rate in children. J Pediatr Surg, 2004; 39: 886–890. [DOI] [PubMed]
- 2).Coursey CA, Nelson RC, Patel MB, Cochran C, Dodd LG, Delong DM, Beam CA, Vaslef S. Making the diagnosis of acute appendicitis: do more preoperative CT scans mean fewer negative appendectomies? A 10-year study. Radiology, 2010; 254: 460–468. [DOI] [PubMed]
- 3).Markar SR, Karthikesalingam A, Cunningham J, Burd C, Bond-Smith G, Kurzawinski TR. Increased use of pre-operative imaging and laparoscopy has no impact on clinical outcomes in patients undergoing appendicectomy. Ann R Coll Surg Engl, 2011; 93: 620–623. [DOI] [PMC free article] [PubMed]
- 4).Styrud J, Eriksson S, Nilsson I, Ahlberg G, Haapaniemi S, Neovius G, Rex L, Badume I, Granström L. Appendectomy versus antibiotic treatment in acute appendicitis. a prospective multicenter randomized controlled trial. World J Surg, 2006; 30: 1033–1037. [DOI] [PubMed]
- 5).Malik AA, Bari SU. Conservative management of acute appendicitis. J Gastrointest Surg, 2009; 13: 996–970. [DOI] [PubMed]
- 6).Hansson J, Körner U, Khorram-Manesh A, Solberg A, Lundholm K. Randomized clinical trial of antibiotic therapy versus appendicectomy as primary treatment of acute appendicitis in unselected patients. Br J Surg, 2009; 96: 473–481. [DOI] [PubMed]
- 7).Vons C, Barry C, Maitre S, Pautrat K, Leconte M, Costaglioli B, Karoui M, Alves A, Dousset B, Valleur P, Falissard B, Franco D. Amoxicillin plus clavulanic acid versus appendicectomy for treatment of acute uncomplicated appendicitis: an open-label, non-inferiority, randomised controlled trial. Lancet, 2011; 377: 1573–1579; commentaries 1545. [DOI] [PubMed]
- 8).Paajanen H, Grönroos JM, Rautio T, Nordström P, Aarnio M, Rantanen T, Hurme S, Dean K, Jartti A, Mecklin JP, Sand J, Salminen P. A prospective randomized controlled multicenter trial comparing antibiotic therapy with appendectomy in the treatment of uncomplicated acute appendicitis (APPAC trial). BMC Surg, 2013; 13: 3. [DOI] [PMC free article] [PubMed]
- 9).Varadhan KK, Neal KR, Lobo DN. Safety and efficacy of antibiotics compared with appendicectomy for treatment of uncomplicated acute appendicitis: meta-analysis of randomised controlled trials. BMJ, 2012; 344: e2156. [DOI] [PMC free article] [PubMed]
- 10).Grönroos JM, Grönroos P. Leucocyte count and C-reactive protein in the diagnosis of acute appendicitis. Br J Surg, 1999; 86: 501–504. [DOI] [PubMed]
- 11).Sand M, Bechara FG, Holland-Letz T, Sand D, Mehnert G, Mann B. Diagnostic value of hyperbilirubinemia as a predictive factor for appendiceal perforation in acute appendicitis. Am J Surg, 2009; 198: 193–198. [DOI] [PubMed]
- 12).Assicot M, Gendrel D, Carsin H, Raymond J, Guilbaud J, Bohuon C. High serum procalcitonin concentrations in patients with sepsis and infection. Lancet, 1993; 341: 515–518. [DOI] [PMC free article] [PubMed]
- 13).Dandona P, Nix D, Wilson MF, Aljada A, Love J, Assicot M, Bohuon C. Procalcitonin increase after endotoxin injection in normal subjects. J Clin Endocrinol Metab, 1994; 79: 1605–1608. [DOI] [PubMed]
- 14).Brunkhorst FM, Heinz U, Forycki ZF. Kinetics of procalcitonin in iatrogenic sepsis. Intensive Care Med, 1998; 24: 888–889. [DOI] [PubMed]
- 15).Becker KL, Nylén ES, White JC, Müller B, Snider RH Jr. Clinical review 167: Procalcitonin and the calcitonin gene family of peptides in inflammation, infection, and sepsis: a journey from calcitonin back to its precursors. J Clin Endocrinol Metab, 2004; 89: 1512–1525. [DOI] [PubMed]
- 16).Christ-Crain M, Jaccard-Stolz D, Bingisser R, Gencay MM, Huber PR, Tamm M, Müller B. Effect of procalcitonin-guided treatment on antibiotic use and outcome in lower respiratory tract infections: cluster-randomised, single-blinded intervention trial. Lancet, 2004; 363: 600–607. [DOI] [PubMed]
- 17).Nakamura A, Wada H, Ikejiri M, Hatada T, Sakurai H, Matsushima Y, Nishioka J, Maruyama K, Isaji S, Takeda T, Nobori T. Efficacy of procalcitonin in the early diagnosis of bacterial infections in a critical care unit. Shock, 2009; 31: 586–591. [DOI] [PubMed]
- 18).Mofidi R, Suttie SA, Patil PV, Ogston S, Parks RW. The value of procalcitonin at predicting the severity of acute pancreatitis and development of infected pancreatic necrosis: systematic review. Surgery, 2009; 146: 72–81. [DOI] [PubMed]
- 19).Riedel S, Melendez JH, An AT, Rosenbaum JE, Zenilman JM. Procalcitonin as a marker for the detection of bacteremia and sepsis in the emergency department. Am J Clin Pathol, 2011; 135: 182–189. [DOI] [PubMed]
- 20).Hattori T, Nishiyama H, Kato H, Ikegami S, Nagayama M, Asami S, Usami M, Suzuki M, Murakami I, Minoshima M, Yamagishi H, Yuasa N. Clinical value of procalcitonin for patients with suspected bloodstream infection. Am J Clin Pathol, 2014; 141: 43–51. [DOI] [PubMed]
- 21).Blab E, Kohlhuber U, Tillawi S, Schweitzer M, Stangl G, Ogris E, Rokitansky A. Advancements in the diagnosis of acute appendicitis in children and adolescents. Eur J Pediatr Surg, 2004; 14: 404–409. [DOI] [PubMed]
- 22).Kafetzis DA, Velissariou IM, Nikolaides P, Sklavos M, Maktabi M, Spyridis G, Kafetzis DD, Androulakakis E. Procalcitonin as a predictor of severe appendicitis in children. Eur J Clin Microbiol Infect Dis, 2005; 24: 484–487. [DOI] [PubMed]
- 23).Chakhunashvili L, Inasaridze A, Svanidze S, Samkharadze J, Chkhaidze I. Procalcitonin as the biomarker of inflammation in diagnostics of pediatric appendicular peritonitis and for the prognosis of early postoperative complications. Georgian Med News, 2005; 129: 78–81. [PubMed]
- 24).Sand M, Trullen XV, Bechara FG, Pala XF, Sand D, Landgrafe G, Mann B. A prospective bicenter study investigating the diagnostic value of procalcitonin in patients with acute appendicitis. Eur Surg Res, 2009; 43: 291–297. [DOI] [PMC free article] [PubMed]
- 25).Kaya B, Sana B, Eris C, Karabulut K, Bat O, Kutanis R. The diagnostic value of D-dimer, procalcitonin and CRP in acute appendicitis. Int J Med Sci, 2012; 9: 909–915. [DOI] [PMC free article] [PubMed]
- 26).Yu CW, Juan LI, Wu MH, Shen CJ, Wu JY, Lee CC. Systematic review and meta-analysis of the diagnostic accuracy of procalcitonin, C-reactive protein and white blood cell count for suspected acute appendicitis. Br J Surg, 2013; 100: 322–329. [DOI] [PubMed]
- 27).DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics, 1988; 44: 837–845. [PubMed]
- 28).Blomqvist PG, Andersson RE, Granath F, Lambe MP, Ekbom AR. Mortality after appendectomy in Sweden, 1987–1996. Ann Surg, 2001; 233: 455–460. [DOI] [PMC free article] [PubMed]
- 29).Paajanen H, Kettunen J, Kostiainen S. Emergency appendectomies in patients over 80 years. Am Surg, 1994; 60: 950–953. [PubMed]
- 30).Anielski R, Kuśnierz-Cabala B, Szafraniec K. An evaluation of the utility of additional tests in the preoperative diagnostics of acute appendicitis. Langenbecks Arch Surg, 2010; 395: 1061–1068. [DOI] [PubMed]
- 31).Tschuor C, Raptis DA, Limani P, Bächler T, Oberkofler CE, Breitenstein S, Graf R. The value of pancreatic stone protein in predicting acute appendicitis in patients presenting at the emergency department with abdominal pain. BMC Gastroenterol, 2012; 12: 154. [DOI] [PMC free article] [PubMed]
- 32).Brănescu C, Şerban D, Şavlovschi C, Dascălu AM, Kraft A. Lipopolysaccharide binding protein (L.B.P.)--an inflammatory marker of prognosis in the acute appendicitis. J Med Life, 2012; 5: 342–347. [PMC free article] [PubMed]
- 33).Nemeth L, Reen DJ, O’Briain DS, McDermott M, Puri P. Evidence of an inflammatory pathologic condition in “normal” appendices following emergency appendectomy. Arch Pathol Lab Med, 2001; 125: 759–764. [DOI] [PubMed]
- 34).Roberts JK, Behravesh M, Dmitrewski J. Macroscopic findings at appendicectomy are unreliable: implications for laparoscopy and malignant conditions of the appendix. Int J Surg Pathol, 2008; 16: 386–390. [DOI] [PubMed]
- 35).Hussain A, Mahmood H, Singhal T, Balakrishnan S, El-Hasani S. What is positive appendicitis? A new answer to an old question. Clinical, macroscopical and microscopical findings in 200 consecutive appendectomies. Singapore Med J, 2009; 50: 1145–1149. [PubMed]
- 36).Carr NJ. The pathology of acute appendicitis. Ann Diagn Pathol. 2000; 4: 46–58. [DOI] [PubMed]
- 37).J Rosai. Rosai and Ackerman’s Surgical Pathology, 10th edition. pp. 714–716, 2011, Mosby, Saint Louis.
- 38).Christ-Crain M, Jaccard-Stolz D, Bingisser R, Gencay MM, Huber PR, Tamm M, Müller B. Effect of procalcitonin-guided treatment on antibiotic use and outcome in lower respiratory tract infections: cluster-randomised, single-blinded intervention trial. Lancet, 2004; 363: 600–607. [DOI] [PubMed]
- 39).Oláh A, Belágyi T, Issekutz A, Makay R, Zaborszky A. Value of procalcitonin quick test in the differentiation between sterile and infected forms of acute pancreatitis. Hepatogastroenterology, 2005; 52: 243–245. [PubMed]
- 40).Carrol ED, Thomson AP, Hart CA. Procalcitonin as a marker of sepsis. Int J Antimicrob Agents, 2002; 20: 1–9. [DOI] [PubMed]
- 41).Aslan A, Karaveli C, Ogunc D, Elpek O, Karaguzel G, Melikoglu M. Does noncomplicated acute appendicitis cause bacterial translocation? Pediatr Surg Int, 2007; 23: 555–558. [DOI] [PubMed]