Abstract
Objective
We investigated the association between number of prenatal visits (PNV) and pregnancy outcomes.
Study Design
A retrospective cohort of 12,092 consecutive, uncomplicated term births was included. Exclusion criteria included unknown or 3rd trimester pregnancy dating, pre-existing medical conditions, and common pregnancy complications. Patients with ≤10 PNV were compared to those with > 10. The primary outcome was a neonatal composite including NICU admission, low APGAR score (<7), low umbilical cord pH (<7.10), and neonatal demise. Secondary outcomes included components of the composite as well as vaginal delivery, induction and cesarean delivery. Logistic regression was used to adjust for potential confounders.
Results
Of 7256 patients in the cohort meeting inclusion criteria, 30% (N=2163) had >10 PNV and the remaining 70% (N=5093) had ≤10. There was no difference in the neonatal composite between the two groups. However, women with > 10 PNV were more likely to undergo induction of labor and cesarean delivery.
Conclusion
Low-risk women with ≥ 10 PNV had higher rates of pregnancy interventions without improvement in neonatal outcomes.
INTRODUCTION
Prenatal care in the United States is widely accepted as an important public health intervention; yet, its efficacy remains largely unstudied and unproven.1, 2 The United States Public Health Service (USPHS) convened a multidisciplinary panel in 1989, the Expert Panel on the Content of Prenatal Care,1 which called for a reduced prenatal visit schedule for healthy, low-risk women based on expert opinion and a review of the literature. A randomized trial of randomized 2764 low-risk women comparing routine care to the reduced USPHS prenatal visit schedule found an average of 2.7 fewer visits in the experimental group without a significant increase in preeclampsia, cesarean delivery, low birth weight or patient satisfaction. More recently, a Cochrane Review found that, in high income countries, there were no differences in perinatal mortality between women randomized to higher vs. reduced prenatal visit (PNV) care groups, but low- and middle-income countries had significantly higher rates of perinatal mortality in women with a reduced PNV schedule.6
The current recommended American Congress of Obstetrics and Gynecology (ACOG) prenatal visit schedule for uncomplicated first pregnancies consists of a visit every 4 weeks until 28 weeks, every 2 weeks until 36 weeks, and weekly until delivery.7 Historically, back-loading the majority of visits in the third trimester was done to detect maternal signs and symptoms of preeclampsia.3 However, available data do not show whether this schedule, or any other prenatal visit schedule, is adequate to improve maternal and neonatal outcomes. Many European countries generally have fewer prenatal visits (PNV) with better birth outcomes compared to the United States.8 For example, the median number of PNV in the United States is 11 compared to 7 in France; yet, the infant mortality rate in France is 3.1/1000 while it is 6.1/1000 in the United States.9 These numbers suggest more PNV may not necessarily mean better outcomes.
We studied the associations between rates of prenatal visit utilization and pregnancy outcomes in a low-risk population who delivered at term. We hypothesized that low-risk women with high prenatal care utilization would be more likely to have pregnancy interventions, such as induction of labor and cesarean delivery, with no commensurate improvement in neonatal outcomes. We posit that prenatal care, in its most basic form, is a surveillance system for low-risk women that works well up to a certain threshold. When a low-risk woman exceeds this critical point, she is potentially exposed to additional testing which possibly leads to unnecessary interventions.
MATERIALS AND METHODS
This was a retrospective cohort study of all consecutive women who were admitted to the Barnes Jewish Hospital Labor and Delivery unit from 2004-2008. Institutional review board approval was obtained from Washington University School of Medicine. Women were eligible if they delivered a pregnancy during the study period and had pregnancies dated by first or second trimester ultrasound at our institution or another institution with an available report. Trimester of dating served as a proxy for when they initiated care. Maternal exclusion criteria included pre-existing medical condition (i.e. diabetes, chronic hypertension or asthma) or complications that developed during the pregnancy (fetal anomaly, multiple gestation, delivery prior to 37 weeks, gestational hypertension, preeclampsia, and gestational diabetes). Patients with an unknown number of prenatal visits or no prenatal care were also excluded from the study. Trained obstetrics research assistants extracted information on maternal demographics, medical history, antepartum course, labor and delivery records and neonatal outcomes from the medical record.
The prenatal visit variable was categorical and divided into > 10 visits, 6-10 visits or ≤5 visits. Based on a prior study by Buekens8 that found the median number of PNV in the United States was 11, we selected >10 visits as the cut-off for high utilization. Our primary outcome was a neonatal composite including NICU admission, low 5 minute APGAR score (<7), low umbilical artery cord pH < 7.10 or neonatal demise. Secondary neonatal outcomes included components of the composite as well as small for gestational age (birthweight<10%) while secondary maternal outcomes included vaginal delivery, induction of labor and cesarean delivery.
Gestational age was calculated by last menstrual period (LMP) if the first trimester ultrasound confirmed the due date within 7 days or a second trimester ultrasound confirmed the due date within 10 days. Otherwise, the pregnancy was redated according to the earliest ultrasound available.10
Since patients who delivered early term had fewer weeks to utilize prenatal visits, we used time-to-event analysis to account for gestational age at delivery. The Cox proportional hazard model was fitted to estimate hazard ratios (HRs), adjusting for potentially confounding factors, including Medicaid insurance, obesity, and nulliparity. The proportional hazards assumption was tested using Schoenfeld's global test.
Data analysis was performed with descriptive and bivariate statistics with the unpaired Student's t- test or Mann-Whitney U test for continuous variables and Chi-square or Fisher exact test for categorical variables. Normality of distribution was tested with the Kolmogorov-Smirnov test. Multi-variable logistic regression models for outcomes of interest were developed to estimate the impact of a more intensive PNV schedule after adjusting for potential confounders. Relevant covariates for inclusion in the initial multivariable statistical models were selected based on the results of the stratified analyses. Factors were removed in a backward stepwise fashion, based on significant changes in the adjusted odds ratio. The final models were adjusted for early term birth (37.0-38.9 weeks), Medicaid insurance status, obesity (body mass index [BMI] ≥ 30kg/m2) and nulliparity. All models were tested with the Hosmer-Lemeshow goodness-of-fit test. We assessed the degree of missing values for each variable of interest for patients meeting eligibility criteria. We did not account for missing data in the final analysis because the data for each variable in the study was >96% complete in patients meeting eligibility criteria for the study.
The statistical analysis was performed with STATA software (version 11, College Station, TX).
RESULTS
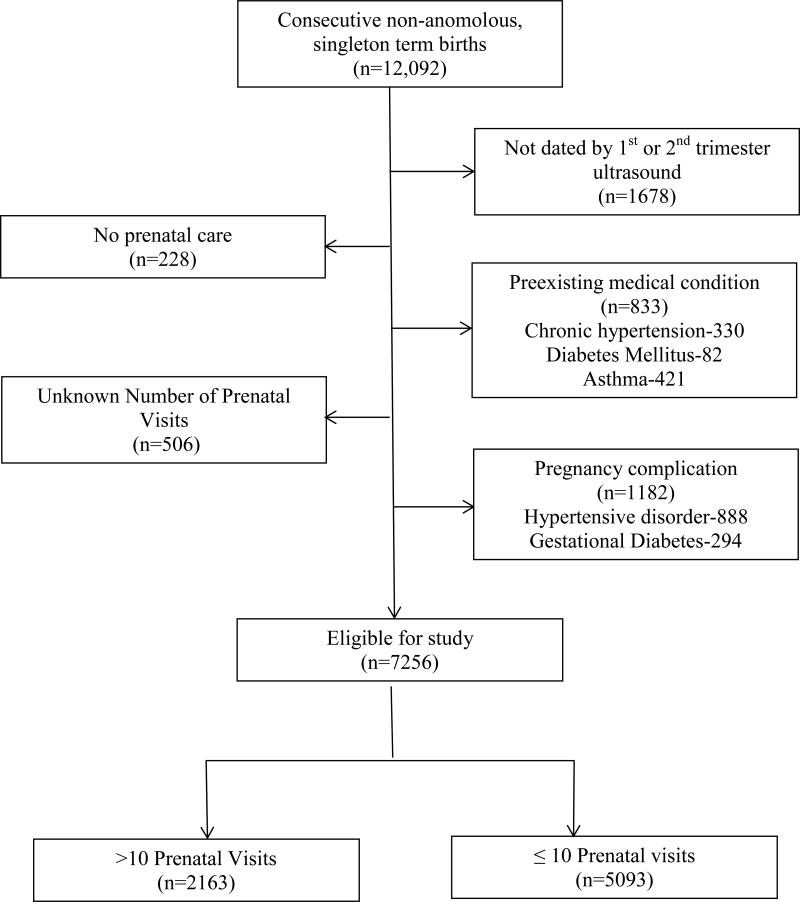
Of 12,092 consecutive women, 1678 were excluded because they were not dated by a 1st or 2nd trimester ultrasound, 506 were excluded for unknown number of PNV and 228 were excluded because they had no prenatal care. Of the remaining women, 833 were excluded for pre-existing medical conditions and 1182 were excluded for pregnancy complications. The remaining 7256 (60%) patients were included in the final analysis (Figure 1). Of these, 30% (N=2163) had > 10 PNV and the remaining 70% (N=5093) had 10 or fewer. Women who were excluded from the analysis for unknown or 3rd trimester dating were more likely to be younger (median age 23 vs. 24 years; p<0.001), African American (80% vs. 60%; p<0.001), uninsured (6% vs. 3%; p<0.001), have a prior preterm birth (12% vs. 9%; p=0.001), and use alcohol (2% vs. 1%; p=0.001) or tobacco (22% vs. 15%; p<0.001) than women in the study with earlier dating.
Figure 1.
Flowchart of study population
High prenatal care utilizers were more likely to be older with 1st trimester dating and obesity while low utilizers were more likely to be African-American, on Medicaid, nulliparous, married, use tobacco and deliver early term (Table 1). Rates of advanced maternal age (AMA) > 35 years old, lack of insurance, prior cesarean, prior preterm birth and alcohol use were similar between groups (Table 1).
Table 1.
Baseline demographics by PNV utilization in healthy women delivering at term with 1st or 2nd trimester dating
| ≤ 10 PNV N=5093 | > 10 PNV N=2163 | p | |
|---|---|---|---|
| Maternal age, years (median, IQR) | 24 (20-29) | 24 (21-29) | 0.005 |
| Advance maternal age ≥ 35 yr | 351 (6.89) | 176 (8.14) | 0.062 |
| Age < 18yrs | 323 (6.34) | 173 (8.00) | 0.011 |
| Dating | |||
| 1st trimester ultrasound | 1103 (21.66) | 647 (29.91) | <0.001 |
| 2nd trimester ultrasound | 3,990 (78.34) | 1516 (70.09) | |
| Early Term Delivery 37-38 wks | 1633 (32.06) | 559 (25.84) | <0.001 |
| Gestational age at delivery in weeks -Mean (SD) | 39.02 (1.17) | 39.26 (1.18) | <0.001 |
| African-American race | 3444 (67.62) | 1349 (62.37) | <0.001 |
| Medicaid or Uninsured | 4164 (81.76) | 1660 (76.75) | <0.001 |
| Married | 641 (12.59) | 218 (10.08) | 0.002 |
| Obese (BMI ≥ 30kg/m2) | 2704 (54.24) | 1293 (60.99) | <0.001 |
| Nulliparous | 906 (17.79) | 310 (14.33) | <0.001 |
| Prior cesarean | 523 (10.27) | 212 (9.80) | 0.546 |
| Prior Preterm Birth | 453 (8.9) | 172 (7.96) | 0.193 |
| Labor type | |||
| Spontaneous | 1761 (34.58) | 600 (29.08) | |
| Augmented | 1587 (31.16) | 639 (30.97) | <0.001 |
| Induced | 1577 (30.96) | 824 (39.94) | |
| Unknown | 168 (3.30) | 100 (4.62) | |
| Regional anesthesia | 1914 (88.04) | 556 (91.00) | 0.041 |
| Alcohol | 55 (1.08) | 22 (1.02) | 0.811 |
| Tobacco | 814 (15.98) | 259 (11.97) | <0.001 |
There was no difference in the primary neonatal composite outcome between high vs. low utilization groups (adjusted odds ratio [aOR] 1.24; 95% confidence interval [CI] 0.94-1.63) or in the individual components of NICU admission, 5 minute APGAR score < 7, neonatal demise or small for gestational age. There were significant differences in secondary maternal outcomes based on number of prenatal visits. The highest utilizers of prenatal care were 33% more likely to be induced (aOR 1.33; 95% CI 1.20-1.49). They were also 31% less likely to have a vaginal delivery (aOR 0.69; 95% CI 0.59-0.76) and 50% more likely to have a cesarean (aOR 1.50; 95% CI 1.32-1.69). (Table 2) Of note, the baseline cesarean section rate and induction rates of the 12,092 women initially screened for this study were 20% and 36% respectively. The leading reason for induction, which occurred in 2401/7256 (33%) women in the study cohort was “elective” in both groups, but was significantly higher in the high vs. low utilization group (49% vs. 42%; p<0.001). Additional reasons for induction were not significantly different between the high and low utilization groups, including “other” (20% vs. 22%; p=0.219), premature rupture of membranes (14% vs. 16%; p=0.129), oligohydramnios (11% vs. 11%; p=0.683) and comorbidity (4% vs. 4%; p=0.851).
Table 2.
Risk of outcomes by prenatal visit number in healthy women delivering at term with 1st or 2nd trimester dating
| ≤ 10 PNV n=5093 n (%) | > 10 PNV n=2163 n (%) | Unadjusted RR (95% CI) | Adjusted OR* (95% CI) | |
|---|---|---|---|---|
| Primary Outcome | ||||
| Neonatal composite | 157 (3.08) | 83 (3.84) | 1.25 (0.96-1.64) | 1.24 (0.94-1.63) |
| Secondary Fetal Outcomes | ||||
| NICU admission | 41 (0.81) | 16 (0.74) | 0.92 (0.52-1.63) | 0.97 (0.54-1.74) |
| 5 min APGAR<7 | 77 (1.51) | 40 (1.85) | 1.22 (0.84-1.79) | 1.23 (0.84-1.82) |
| pH < 7.10 | 82 (1.62) | 48 (2.23) | 1.37 (0.97-2.00) | 1.35 (0.94-1.94) |
| Neonatal Demise | 1 (0.05) | 0 | -- | -- |
| Small for gestational age | 222 (10.26) | 629 (12.35) | 0.81 (0.69-0.95) | 0.91 (0.77-1.07) |
| Secondary Maternal Outcomes | ||||
| Induction of Labor | 1577 (30.96) | 824 (38.10) | 1.23 (1.15-1.32) | 1.33 (1.20-1.49) |
| Vaginal Delivery | 4194 (82.35) | 1624 (75.08) | 0.65 (0.57-0.73) | 0.69 (0.59-0.76) |
| Cesarean Delivery | 899 (17.65) | 539 (24.92) | 1.41 (1.28-1.55) | 1.50 (1.32-1.69) |
Adjusted for early term birth, medicaid insurance, obesity and nulliparity
We used a time-to-event analysis to account for the effect of gestational age at delivery on the primary outcome since patients who delivered early term had fewer weeks to experience prenatal visits. After adjusting for confounders in a Cox proportional hazard model, the risk for the neonatal composite (HR 1.05; 95%CI 0.80-1.38) was still not significantly different between the high and low prenatal visit utilization groups.
DISCUSSION
Our general clinical approach to prenatal care in the United States is that early is better than late, some is better than none, and more is better than less. Our findings challenge this traditional line of thinking among a population of low-risk, healthy women. We found that there was no evidence of a reduced risk of adverse neonatal outcomes among women with the highest utilization of prenatal care; however, they were more likely to undergo interventions including labor induction and cesarean.
Prenatal care was intended to mitigate the complications of preeclampsia, and later to reduce the risk of low birth weight, preterm birth and the associated morbidity/mortality3 There is certainly a role for surveillance through prenatal care in order to identify and manage women with pregnancy complications, but we may need to be more innovative in our approach.
Several indices to evaluate the adequacy of prenatal care have been developed, including the Kessner Index, the R-GINDEX and Kotelchuck's Adequacy of Prenatal care utilization. 12-14 Each uses a combination of the month when prenatal care was initiated and/or the number of prenatal visits to estimate whether prenatal care was sufficient, largely based on adherence to professional society guidelines; but, none was designed to assess whether adherence translates into meaningful outcomes for mothers or babies.
A study in Massachusetts of the Healthy Start expansion of prenatal coverage from 1984-1987 showed women with “satisfactory care” based on the Kessner Index14 had lower rates of adverse maternal outcomes including, pregnancy-induced hypertension, placental abruption and hospital stay exceeding their infant's length of stay, than those with “inadequate” care; however, the increased health coverage did not improve prenatal care utilization or birth outcomes and actually increased the rates of cesarean delivery.11 The authors were unsure of whether the increased cesarean rate represented an improvement or worsening of care quality; but, suggested that payer was a determinant of cesarean rate independent of clinical factors.
A strength of our study is that it provides an update to the literature on PNV utilization, most of which was done in the 1970-1990s, in a large cohort of 7256 women. This study focuses on assessing rates of prenatal care utilization to optimize maternal and fetal outcomes rather than adherence to a prescribed visit schedule.
However, our study is not without limitations. Our cut-off of 10 PNV is actually lower than the recommended ACOG prenatal schedule for patients who initiate care in the first trimester.7 For example, a woman who initiates care at 12 weeks and delivers at 39 weeks should have 12 PNV based on this schedule. The top quartile in our population falls below this threshold, which is reflective of the fact that the majority of our patients initiate care in the 2nd trimester. We chose to include patients dated in the 2nd trimester for the sake of generalizability since 78% of our study population would have been eliminated if only patients dated by 1st trimester ultrasound were included. We used the number of PNV as a proxy for prenatal care utilization, but counting visits neither accounts for the quality nor content of the visit. Therefore, this method likely oversimplifies the scope of antepartum care received by the patients. The number of possible prenatal visits in a pregnancy is impacted by the gestational age at which prenatal care is initiated, the timing of delivery (pre-term deliveries inherently have fewer visits), need for hospitalization (with resulting missed visits while an inpatient), pregnancy complications and patient compliance. We attempted to address this limitation, at least in part, through a time to event analysis and by only including women who initiated care in the first two trimesters (as evidenced by the timing of their dating ultrasound), delivered on or after 37 weeks, and did not have common pre-existing medical conditions or pregnancy complications. Thus, despite the fact we are a tertiary referral center with a high level of acuity, our exclusion criteria should make our results more generalizable to a low-risk population, although we acknowledge that our largely poor, obese African American population with a tendency to initiate care later in pregnancy is still likely higher risk than the general population.
Our sample represented 60% of patients among all consecutive, non-anomalous singletons delivering at our tertiary care referral center during the study period. Unfortunately, one cannot prospectively know whether a woman will develop a pregnancy complication, and prenatal care is at least in part intended to detect pregnancy complications; thus, our cohort cannot be completely identified until postpartum. We acknowledge that there are a multitude of co-morbidities, prior and current pregnancy complications that may deem a pregnancy high risk and we have only captured the most common in our study; thus, it is important to consider the possibility of selection bias. Some higher risk women may be included in our cohort and are more likely to be represented in the high utilization group due to more intensive antenatal surveillance with increased risk for intervention and poor outcomes. However, the fact that both the high and low-utilization groups had equal rates of reason for induction listed as “comorbidities” (4% in both groups compared to 8% listed as reason for induction among 4355/12,092 patients induced in general population), suggests there were a similar number of women with comorbidities we were unable to capture in both groups. Lastly, by excluding patients who delivered preterm, we were unable to determine how the number of PNV may impact outcomes in this population, which is very important from a neonatal morbidity/mortality perspective.
This study suggests an association between high prenatal care utilization and pregnancy interventions, but the cause-effect relationship remains unclear. These women could be the “worried-well” driving their own care, have subtle issues causing their providers to schedule more visits, or they could represent a general population adhering to the ACOG prenatal visit schedule with each additional visit (perhaps beyond a certain threshold) increasing their risk for intervention. Further research is needed to aid in the rational design of prenatal care to optimize maternal and neonatal outcomes in a prospective manner with a study sample that is powered to elucidate important differences such as preterm birth rates. We also believe that studying alternative methods of prenatal care, such as enhanced care models with an intensive educational component, or group prenatal care through CenteringPregnancy 15, 16, are necessary to discover innovative means of caring for women in an evidence-based manner.
Prenatal care delivery in its current form became the standard of care before any randomized controlled trials were conducted to prove efficacy. The importance of this public health intervention is now so widely accepted that it is difficult to study in a prospective manner or randomize patients to different frequencies or types of care. Our findings suggest that the widely accepted, but largely unstudied idea that more PNV produce better outcomes does not necessarily hold.
Supplementary Material
Acknowledgments
DISCLOSURE STATEMENT: Dr. Carter is supported by a NIH T32 training grant (5T32HD055172-05). Dr. Cahill was a Robert Wood Johnson Foundation Physician Faculty Scholar, which partially supported this work.
Footnotes
CONFLICT OF INTEREST:
The authors report no conflicts of interest.
REFERENCES
- 1.Rosen MG, Merkatz IR, Hill JG. Caring for Our Future: A report by the Expert Panel on the Content of Prenatal Care. Obstetrics and Gynecology. 1991;77(5):782–7. [PubMed] [Google Scholar]
- 2.Vlllar J. Scientific basis for the content of routine antenatal care I. Philosophy, recent studies, and power to eliminate or alleviate adverse maternal outcomes. Acta Obstetricia et Gynecologica Scandinavica. 1997;76(1):1–14. doi: 10.3109/00016349709047778. [DOI] [PubMed] [Google Scholar]
- 3.Alexander GR, Kotelchuck M. Assessing the role and effectiveness of prenatal care: History, challenges, and directions for future research. Public Health. 2002;116(4):306–16. doi: 10.1016/S0033-3549(04)50052-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McDuffie RS, Jr, Beck A, Bischoff K, Cross J, Orleans M. Effect of frequency of prenatal care visits on perinatal outcome among low-risk women: A randomized controlled trial. Journal of the American Medical Association. 1996;275(11):847–51. [PubMed] [Google Scholar]
- 5.Petrou S, Kupek E, Vause S, Maresh M. Antenatal visits and adverse perinatal outcomes: Results from a British population-based study. European Journal of Obstetrics Gynecology and Reproductive Biology. 2003;106(1):40–9. doi: 10.1016/s0301-2115(02)00215-4. [DOI] [PubMed] [Google Scholar]
- 6.Dowswell T, Carroli G, Duley L, et al. Alternative versus standard packages of antenatal care for low-risk pregnancy. Cochrane database of systematic reviews (Online) 2010;(10) doi: 10.1002/14651858.CD000934.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.American Academy of Pediatrics ACoO, Gynecologists Guidelines for perinatal care. 2012 http://search.ebscohost.com/login.aspx?direct=true&scope=site&db=nlebk&db=nlabk&AN=567165.
- 8.Buekens P, Kotelchuck M, Blondel B, Kristensen FB, Chen JH, Masuy-Stroobant G. A comparison of prenatal care use in the United States and Europe. American Journal of Public Health. 1993;83(1):31–6. doi: 10.2105/ajph.83.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.MacDorman MF, Matthews TJ, Mohangoo AD, Zeitlin J. International comparisons of infant mortality and related factors: United States and Europe, 2010. National vital statistics reports : from the Centers for Disease Control and Prevention, National Center for Health Statistics, National Vital Statistics System. 2014;63(5):1–6. [PubMed] [Google Scholar]
- 10.ACOG Practice Bulletin No. 101: Ultrasonography in Pregnancy. Obstetrics & Gynecology. 2009;113(2, Part 1):451–61. doi: 10.1097/AOG.0b013e31819930b0. 10.1097/AOG.0b013e31819930b0. [DOI] [PubMed] [Google Scholar]
- 11.Haas JS, Udvarhelyi S, Epstein AM. The effect of health coverage for uninsured pregnant women on maternal health and the use of cesarean section. Journal of the American Medical Association. 1993;270(1):61–4. [PubMed] [Google Scholar]
- 12.Kogan MD, Martin JA, Alexander GR, Kotelchuck M, Ventura SJ, Frigoletto FD. The changing pattern of prenatal care utilization in the United States, 1981-1995, using different prenatal care indices. Journal of the American Medical Association. 1998;279(20):1623–8. doi: 10.1001/jama.279.20.1623. [DOI] [PubMed] [Google Scholar]
- 13.Kotelchuck M. The Adequacy of Prenatal Care Utilization Index: Its US distribution and association with low birthweight. American Journal of Public Health. 1994;84(9):1486–9. doi: 10.2105/ajph.84.9.1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kotelchuck M. An evaluation of the Kessner Adequacy of Prenatal Care Index and a proposed Adequacy of Prenatal Care Utilization Index. American Journal of Public Health. 1994;84(9):1414–20. doi: 10.2105/ajph.84.9.1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Housden L, Wong ST, Dawes M. Effectiveness of group medical visits for improving diabetes care: A systematic review and meta-analysis. CMAJ. 2013;185(13):E635–E44. doi: 10.1503/cmaj.130053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ickovics JR, Kershaw TS, Westdahl C, et al. Group prenatal care and perinatal outcomes: A randomized controlled trial. Obstetrics and Gynecology. 2007;110(2 I):330–9. doi: 10.1097/01.AOG.0000275284.24298.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.