Figure 4. The role of c-IAP1/2 in CD30-mediated NF-κB activation.
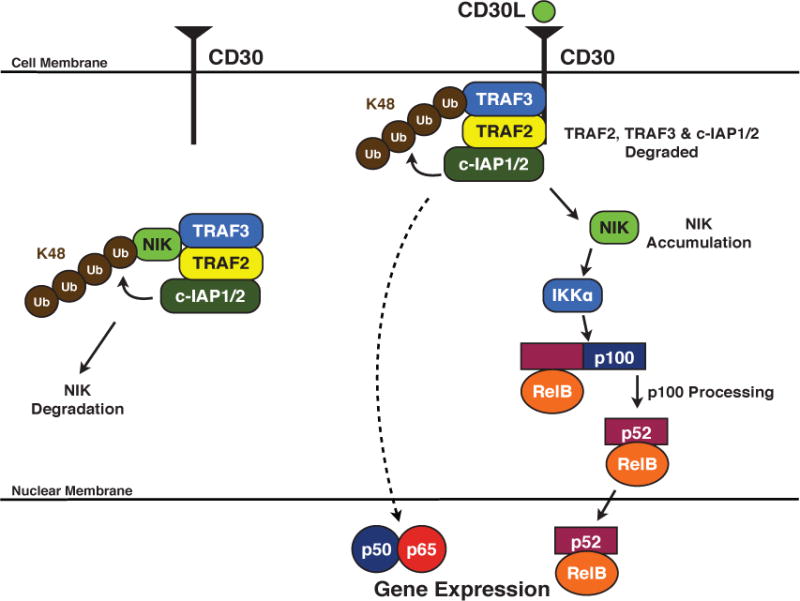
Prior to stimulation, the c-IAPs form a complex with TRAF2 and TRAF3 and ubiquitinate NIK in a K48-dependent manner, resulting in the constitutive degradation of NIK. Following receptor activation, the TRAF:c-IAP complex is recruited to the cytoplasmic tail of CD30, where the c-IAPs ubiquitinate TRAF3, inducing its degradation. TRAF2 and c-IAP1/2 are also degraded, allowing for the accumulation of NIK. NIK activates IKKα, which phosphorylates p100. Subsequently, p100 is cleaved, allowing the active non-canonical NF-κB dimer to translocate into the nucleus. Receptor-mediated canonical NF-κB activation also occurs, albeit through a poorly defined mechanism.
