Abstract
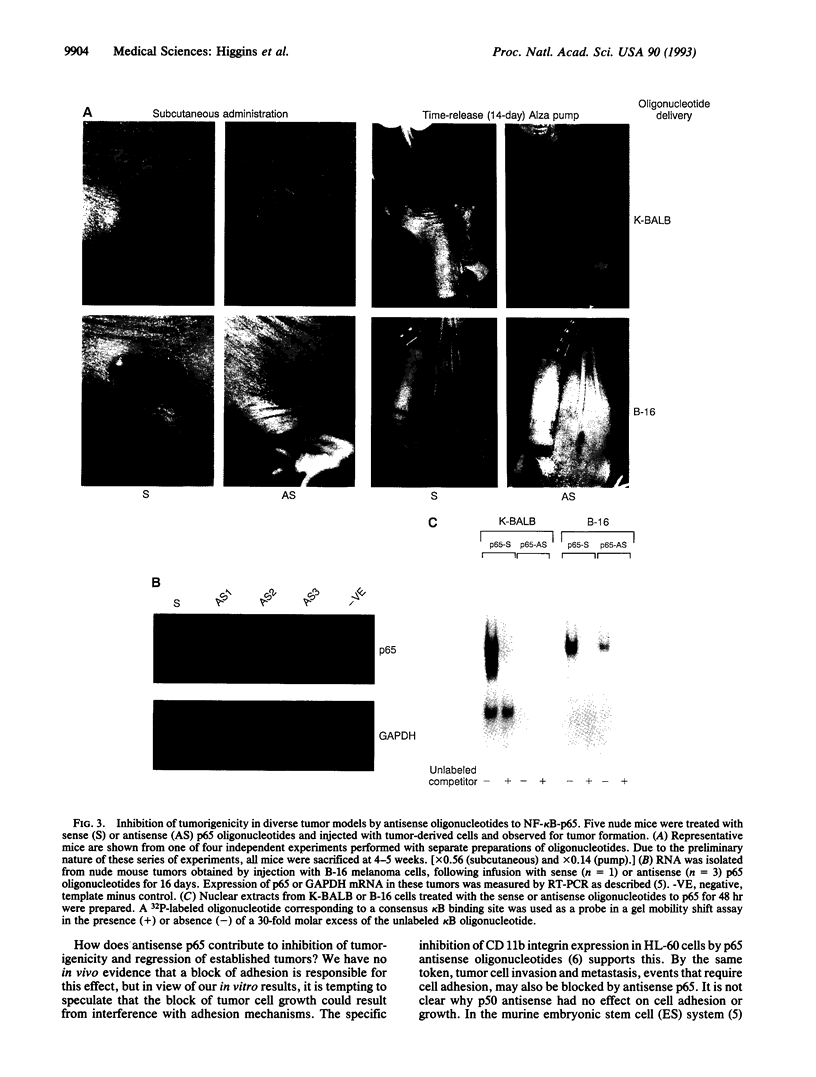
The NF-kappa B transcription factor, composed of two proteins, p50 and p65, is a pleiotropic activator that participates in the induction of a wide variety of cellular genes. Various cell adhesion molecules have NF-kappa B binding sites and may play an important role in inflammatory response, tumorigenicity, and metastasis. In an earlier study, we demonstrated that adhesion of diverse transformed cells was blocked by antisense inhibition of the p65 subunit of NF-kappa B. Since cell-substratum interactions play an important role in tumorigenicity, we reasoned that antisense p65 could inhibit tumorigenicity. In diverse transformed cell lines, phosphorothioate antisense oligonucleotides to p65 inhibited in vitro growth, reduced soft-agar colony formation, and eliminated the ability of cells to adhere to an extracellular matrix. Stable transfectants of a fibrosarcoma cell line expressing dexamethasone-inducible antisense RNA to p65 showed inhibition of in vitro growth and in vivo tumor development. In response to inducible expression of antisense RNA, a pronounced tumor regression was seen in nude mice. The administration of antisense but not sense p65 oligonucleotides caused a pronounced inhibition of tumorigenicity in nude mice injected with diverse tumor-derived cell lines. Inhibitors of NF-kappa B function may thus be useful in the treatment of cancer.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albelda S. M., Buck C. A. Integrins and other cell adhesion molecules. FASEB J. 1990 Aug;4(11):2868–2880. [PubMed] [Google Scholar]
- Calabretta B. Inhibition of protooncogene expression by antisense oligodeoxynucleotides: biological and therapeutic implications. Cancer Res. 1991 Sep 1;51(17):4505–4510. [PubMed] [Google Scholar]
- Cohen J. S. Oligonucleotides as therapeutic agents. Pharmacol Ther. 1991 Nov;52(2):211–225. doi: 10.1016/0163-7258(91)90009-b. [DOI] [PubMed] [Google Scholar]
- Edelman G. M., Crossin K. L. Cell adhesion molecules: implications for a molecular histology. Annu Rev Biochem. 1991;60:155–190. doi: 10.1146/annurev.bi.60.070191.001103. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992 Apr 3;69(1):11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Juliano R. L. Membrane receptors for extracellular matrix macromolecules: relationship to cell adhesion and tumor metastasis. Biochim Biophys Acta. 1987 Nov 25;907(3):261–278. doi: 10.1016/0304-419x(87)90009-6. [DOI] [PubMed] [Google Scholar]
- Kitajima I., Shinohara T., Bilakovics J., Brown D. A., Xu X., Nerenberg M. Ablation of transplanted HTLV-I Tax-transformed tumors in mice by antisense inhibition of NF-kappa B. Science. 1992 Dec 11;258(5089):1792–1795. doi: 10.1126/science.1299224. [DOI] [PubMed] [Google Scholar]
- Liotta L. A., Rao C. N., Wewer U. M. Biochemical interactions of tumor cells with the basement membrane. Annu Rev Biochem. 1986;55:1037–1057. doi: 10.1146/annurev.bi.55.070186.005133. [DOI] [PubMed] [Google Scholar]
- Liotta L. A., Wewer U., Rao N. C., Schiffmann E., Stracke M., Guirguis R., Thorgeirsson U., Muschel R., Sobel M. Biochemical mechanisms of tumor invasion and metastasis. Anticancer Drug Des. 1987 Oct;2(2):195–202. [PubMed] [Google Scholar]
- Matsukura M., Shinozuka K., Zon G., Mitsuya H., Reitz M., Cohen J. S., Broder S. Phosphorothioate analogs of oligodeoxynucleotides: inhibitors of replication and cytopathic effects of human immunodeficiency virus. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7706–7710. doi: 10.1073/pnas.84.21.7706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan R., Higgins K. A., Perez J. R., Coleman T. A., Rosen C. A. Evidence for differential functions of the p50 and p65 subunits of NF-kappa B with a cell adhesion model. Mol Cell Biol. 1993 Jun;13(6):3802–3810. doi: 10.1128/mcb.13.6.3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan R., Klement J. F., Ruben S. M., Higgins K. A., Rosen C. A. Identification of a naturally occurring transforming variant of the p65 subunit of NF-kappa B. Science. 1992 Apr 17;256(5055):367–370. doi: 10.1126/science.256.5055.367. [DOI] [PubMed] [Google Scholar]
- Narayanan R., Lawlor K. G., Schaapveld R. Q., Cho K. R., Vogelstein B., Bui-Vinh Tran P., Osborne M. P., Telang N. T. Antisense RNA to the putative tumor-suppressor gene DCC transforms Rat-1 fibroblasts. Oncogene. 1992 Mar;7(3):553–561. [PubMed] [Google Scholar]
- Narayanan R., Tare N. S., Benjamin W. R., Gubler U. A sensitive technique to monitor gene transfer and expression in bone marrow stem cells. Exp Hematol. 1989 Aug;17(7):832–835. [PubMed] [Google Scholar]
- Neish A. S., Williams A. J., Palmer H. J., Whitley M. Z., Collins T. Functional analysis of the human vascular cell adhesion molecule 1 promoter. J Exp Med. 1992 Dec 1;176(6):1583–1593. doi: 10.1084/jem.176.6.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plantefaber L. C., Hynes R. O. Changes in integrin receptors on oncogenically transformed cells. Cell. 1989 Jan 27;56(2):281–290. doi: 10.1016/0092-8674(89)90902-1. [DOI] [PubMed] [Google Scholar]
- Rao C. N., Castronovo V., Schmitt M. C., Wewer U. M., Claysmith A. P., Liotta L. A., Sobel M. E. Evidence for a precursor of the high-affinity metastasis-associated murine laminin receptor. Biochemistry. 1989 Sep 5;28(18):7476–7486. doi: 10.1021/bi00444a047. [DOI] [PubMed] [Google Scholar]
- Rhim J. S., Park D. K., Arnstein P., Huebner R. J., Weisburger E. K., Nelson-Rees W. A. Transformation of human cells in culture by N-methyl-N'-nitro-N-nitrosoguanidine. Nature. 1975 Aug 28;256(5520):751–753. doi: 10.1038/256751a0. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E. Fibronectin and its receptors. Annu Rev Biochem. 1988;57:375–413. doi: 10.1146/annurev.bi.57.070188.002111. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E., Giancotti F. G. Integrins and tumor cell dissemination. Cancer Cells. 1989 Dec;1(4):119–126. [PubMed] [Google Scholar]
- Ryder K., Lanahan A., Perez-Albuerne E., Nathans D. jun-D: a third member of the jun gene family. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1500–1503. doi: 10.1073/pnas.86.5.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokoloski J. A., Sartorelli A. C., Rosen C. A., Narayanan R. Antisense oligonucleotides to the p65 subunit of NF-kappa B block CD11b expression and alter adhesion properties of differentiated HL-60 granulocytes. Blood. 1993 Jul 15;82(2):625–632. [PubMed] [Google Scholar]
- Stein C. A., Cohen J. S. Oligodeoxynucleotides as inhibitors of gene expression: a review. Cancer Res. 1988 May 15;48(10):2659–2668. [PubMed] [Google Scholar]
- Voraberger G., Schäfer R., Stratowa C. Cloning of the human gene for intercellular adhesion molecule 1 and analysis of its 5'-regulatory region. Induction by cytokines and phorbol ester. J Immunol. 1991 Oct 15;147(8):2777–2786. [PubMed] [Google Scholar]
- Whelan J., Ghersa P., Hooft van Huijsduijnen R., Gray J., Chandra G., Talabot F., DeLamarter J. F. An NF kappa B-like factor is essential but not sufficient for cytokine induction of endothelial leukocyte adhesion molecule 1 (ELAM-1) gene transcription. Nucleic Acids Res. 1991 May 25;19(10):2645–2653. doi: 10.1093/nar/19.10.2645. [DOI] [PMC free article] [PubMed] [Google Scholar]