Figure 2.
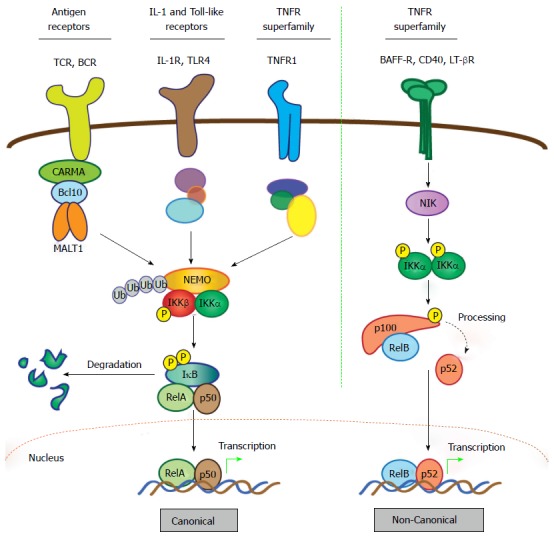
Activation of nuclear factor-κB: Canonical and non-canonical pathways. The NF-κB family comprises five members characterized by the presence of a Rel homology domain, which mediates DNA binding and dimerization. To allow prompt and efficient activation in response to stimuli, NF-κB dimers are sequestered in the cytoplasm in an inactive form via interaction with IκB proteins. Activation of NF-κB quickly follows ligand-induced stimulation of a variety of receptors, including TNF, IL-1, Toll-like, and BCR and TCR. The two principal signaling mechanisms that lead to activation of specific NF-κB heterodimers are the canonical and non-canonical pathways. In the case of canonical signaling, receptor-mediated signaling is transmitted via recruitment and/or activation of various upstream signaling components that promote activation of the IKK complex, which consists of catalytic kinase subunits IKKα and IKKβ and a regulatory scaffold protein called NF-κB essential modulator (NEMO; also called IKKγ). Once the IKK complex is activated, the steps directing canonical NF-κB stimulation are generally shared, regardless of the upstream stimulus. Selectivity is thought to reside mostly in the unique, “private” pathways utilized by distinct receptors for communication with the IKK complex. In the case of antigen receptors, assembly of the CBM signalosome is critical for this to occur. Upon activation of the IKK complex, the IKKβ kinase subunit phosphorylates IκBα, marking it for lysine (K)48-linked ubiquitination and subsequent degradation via the proteasome, which represents the commitment step for canonical NF-κB activation. Newly freed NF-κB heterodimers, most commonly RelA (p65) and p50, translocate to the nucleus where they bind target sites within the promoters of a vast array of responsive genes to induce expression. Notably, the gene encoding IκBα is directly regulated by NF-κB and its synthesis serves as a negative feedback loop, thus attenuating and resetting the canonical NF-κB machinery. In contrast to the multitude of stimuli and signaling intermediates that activate canonical NF-κB, a relatively small number of cell-surface receptors promote non-canonical NF-κB activation. These include the BAFF, LT-β, and CD40 TNF superfamily receptors. Stimulation of non-canonical NF-κB centers on stabilization and activation of the critical upstream regulator, NIK. In resting cells, NIK is targeted for K48-linked ubiquitination and proteasomal degradation by a complex of proteins that includes cIAP1, cIAP2, TRAF2 and TRAF3, which together function as a “destruction complex” and constitutively represses NIK activity. Ligand binding to selective TNF superfamily receptors promotes degradation of TRAF3, the protein which links NIK to cIAP1/2 and TRAF2, thereby freeing NIK from the destruction complex and allowing its stabilization and activation. Then, NIK phosphorylates and activates the IKKα kinase subunit, which in turn phosphorylates the NF-κB2 precursor p100, targeting it for partial proteasomal degradation to p52. Like IκBα, p100 contains C-terminal ankyrin repeats that are responsible for cytoplasmic retention of RelB/p100 complexes in unstimulated cells. Freed RelB/p52 dimers also undergo nuclear translocation and drive transcription of appropriate genes in a manner analogous to the canonical RelA/p50 heterodimer complexes. Unlike canonical NF-κB signal termination, negative regulation of non-canonical NF-κB is less well-characterized. It appears to depend on reciprocal IKKα-dependent phosphorylation of NIK on C-terminal serine residues, which leads to TRAF/cIAP complex-independent NIK destabilization. However, more work is needed to fully define the mechanism involved in attenuating the non-canonical NF-κB signaling pathway. CARMA: CARD and membrane-associated guanylate kinase-like domain-containing protein; NF-κB: Nuclear factor-κB; MALT: Mucosa-associated lymphoid tissue; IκB: Inhibitor of NF-κB; IL: Interleukin; IKK: IκB kinase; BAFF: B-cell activating factor belonging to the TNF family; LT: Lymphotoxin; NIK: NF-κB-inducing kinase; BCR: B-cell receptor; TCR: T-cell antigen receptor.
