Abstract
Worldwide, gastric cancer is one of the most common malignancies with high mortality. Various aspects of the development and progression of gastric cancer continue to be extensively investigated in order to further our understanding and provide more effective means for the prevention, diagnosis, and treatment of the disease. Estrogen receptors (ERs) are steroid hormone receptors that regulate cellular activities in many physiological and pathological processes in different tissues. There are two distinct forms of ERs, namely ERα and ERβ, with several alternative-splicing isoforms for each. They show distinct tissue distribution patterns and exert different biological functions. Dysregulation of ERs has been found to be associated closely with many diseases, including cancer. A number of studies have been conducted to investigate the role of ERs in gastric cancer, the possible mechanisms underlying these roles, and the clinical relevance of deregulated ERs in gastric cancer patients. To date, inconsistent associations of different ERs with gastric cancer have been reported. These inconsistencies may be caused by variations in in vitro cell models and clinical samples, including assay conditions and protocols with regard to different forms of ERs. Given the potential of the deregulated ERs as diagnostic/prognostic markers or therapeutic targets for gastric cancer, it will be important to identify/confirm the association of each ER isoform with gastric cancer, to determine the specific roles and interactions that these individual ER isoforms play under specific conditions in the development and/or progression of gastric cancer, and to elucidate precisely these mechanisms. In this review, we summarize the achievements from early ER studies in gastric cancer to the most up-to-date discoveries, with an effort to provide a comprehensive understanding of the role of ERs roles in gastric cancer and its possible mechanisms. Furthermore, we propose directions for future investigations.
Keywords: Gastric cancer, Estrogen receptor, Isoform, Carcinogenesis, Mechanism, Genomic pathway, Non-genomic pathway
Core tip: Gastric cancer is one of the common malignancies worldwide with high mortality. Estrogen receptors (ERs) are steroid hormone receptors that regulate cellular activities in many physiological and pathological processes of different tissues. Dysregulation of ERs is associated with many diseases, including gastric cancer. Studies have been conducted to investigate the roles that ERs play in gastric cancer and the clinical relevance of deregulated ERs in gastric cancer patients. This review focuses on the current understanding of ERs in gastric cancer and proposes directions for future investigations.
INTRODUCTION
Gastric cancer is one of the most common types of cancer and one of the leading causes of cancer-related deaths worldwide, with an estimated 723100 deaths and 951600 new cases in 2012[1]. Currently, tremendous efforts are being made to investigate the cellular and molecular mechanisms leading to the development and progression of gastric cancer, with the hope of improving the prevention, early diagnosis, and precise and personalized treatment of this cancer.
Estrogens are a class of steroids that was initially found to regulate the development and growth of the human reproductive system. Estrogens exert their influence on specific cells via activation of their cognate receptors (estrogen receptors, ERs). Estrogens/ERs have also been found to be involved in other physiological/pathological processes of cardiovascular, skeletal, and neuroendocrine systems[2]. ERα and ERβ were first cloned from human breast cancer MCF-7 cells and from rat prostate in 1986 and 1996, respectively[3]. ERα and ERβ are members of a superfamily of nuclear receptors that can transduce extracellular signals into transcriptional responses and possess distinct protein structural characteristics, tissue distributions, and functions[4,5]. In certain ligands, cell-types, and promoter contexts, ERα and ERβ have different activities[6].
As illustrated in Figure 1, ERs share conserved domains for ligand binding, DNA binding, transcription activation (AF-1 and AF-2), and nuclear translocation (NLS). Based on their molecular weights, three isoforms for ERα have been identified, designated as ERα66, ERα46, and ERα36[7]. ERα66 functions as a ligand-dependent transcription factor that modulates gene expression by binding to estrogen response elements (EREs) in the transcriptional regulatory region in genomic DNA[5]. Despite lacking the AF-1 activation domain, ERα46 can still bind to EREs and form heterodimers with ERα66[8,9]. The subcellular localization and activation of the Src/phosphoinositide 3 kinase (PI3K)/AKT pathway upon estrogen-signaling also indicate possible roles for ERα46 in non-genomic estrogen signaling[10-12]. For ERα36, both activation domains (AF-1 and AF-2) are absent, but it retains the ability to dimerize, translocate to the nucleus, and bind DNA, with an even broader ligand-binding spectrum, and it may mediate rapid estrogen signaling[7,13]. Five alternatively spliced isoforms for ERβ have been identified (ERβ1-ERβ5). ERβ appears to have a weaker corresponding AF-1 domain, and its transcriptional activation function depends more on the AF-2 domain rather than AF-1 domain.
Figure 1.
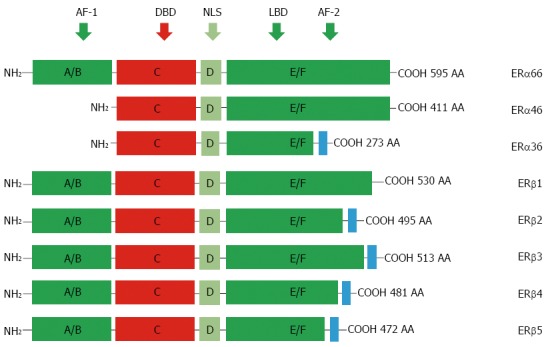
Protein structure of estrogen receptors. Two different forms of ER are encoded by two distinct genes located in chromosomes 6 and 14 and produce two proteins with 595 and 530 amino acids in full length, respectively. Six evolutionary conserved domains, namely A-F, are shared by different ERs. For ERα isoforms, compared to the full length ERα66, ERα46 lacks AF-1 domain (A/M), ERα36 lacks AF-1 and partial AF-2 domains but is equipped an extra different C-terminal. For alternatively-spliced ERβ isoforms, they differ mainly at their C-terminals. AF-1: Transcriptional activation factor-1; DBD: DNA-binding domain; NLS: Nuclear localization signals; LBD: Ligand binding domain; ERs: Estrogen receptors; AA: Amino acid.
Expression of ERs has been well documented in a variety of human tumors, including gastric cancer[14-24]. Hormonal therapy targeting ERs for the treatment of breast cancer has played a remarkable role[25,26]. Investigation on the roles and mechanisms of ERs in gastric cancer will surely provide us additional means for the management of the disease.
In this review, we summarize the achievements from early ERs studies in gastric cancer to the most up-to-date discoveries, with an effort to provide a comprehensive understanding of the role of ERs in gastric cancer and its possible mechanisms. Furthermore, we propose directions for future investigations.
EXPRESSION AND FUNCTIONAL INVESTIGATIONS ON ESTROGEN RECEPTORS IN GASTRIC CANCER
ERα66 (ERα)
Most of the literatures use terms “ER” or “ERα” when actually referring to the specific isoform ERα66, as it was the first identified isoform. Early investigation on ER expression in gastric cancer was initiated by the observation that there was an association between breast cancer and gastrointestinal cancer but no correlation between ER expression and gastric cancer[27]. The ER positive rate in gastric carcinoma was not significantly different between male and female cases[27,28], but the incidence of poorly differentiated adenocarcinoma was significantly higher than that of well differentiated adenocarcinoma[28]. Later studies showed a correlation between ER status and tumor grades in gastric cancer[29,30], and ER expression was found to be associated with diffuse type gastric cancer and shorter disease free survival[31]. At the mRNA level, the ERα expression between gastric cancer tissues and matched normal tissues was not significantly different, but ERα-positive expression was correlated with poorer overall survival[15].
However, results from established cell lines were inconsistent. More cell lines showed ERα expression by real-time polymerase chain reaction (RT-PCR) than that by western blotting[31,32], which may be due to differences in the sensitivity between the assays. ERα overexpression significantly inhibited cell growth and proliferation, promoted cell apoptosis, and blocked cell entry into the G1/G0 phase. In addition, ERα reduced the motility and invasion of gastric cancer cells. Overexpression of ERα decreased β-catenin expression, suggesting that ERα overexpression inhibited cell growth and cancer progression in gastric cancer by attenuating the expression of β-catenin[33].
ERα36
Although no work has been reported on ERα46 in gastric cancer to date, some studies have investigated the clinical significance and functions of ERα36 in gastric cancer. ERα36 is highly expressed in human gastric cancer, and its expression is correlated with lymph node metastasis, suggesting its use as a predictive marker for lymph node metastasis of gastric cancer[34]. Both mRNA and protein of ERα36 were detected in the established gastric cancer cell lines examined. Higher ERα36 mRNA levels were expressed in tumor specimens than in paired normal tissues. ERα36 protein was mainly expressed on the plasma membrane and in the cytoplasm of the established gastric cancer cells[34].
ERβ
Regarding the clinical relevance of ERβ with gastric cancer, the ERβ-positive group was associated with lower tumor stage, negative perineural invasion, Lauren’s intestinal type, and free of recurrence. Presence of ERβ in gastric cancer could have a protective effect against the invasiveness of gastric cancer[32], similar to the function of ERβ in inhibiting proliferation, invasion, and tumor formation of breast cancer cells[35-37]. In multivariate analysis, the absence of ERβ was a significant independent prognostic factor that was associated with poor overall survival[15].
A more recent study, however, showed that although ERs are present in both gastric tumors and normal tissues, their expression levels were extremely low, except for the predominance of ERβ, and they may only be partly involved in gastric carcinogenesis. These data suggest that their clinicopathological and prognostic significance in gastric cancer may be limited[38].
For the transcription variants of ERβ in gastric cancer tissues, higher ERβ5 mRNA level was correlated with pTNM stage of the tumor, and lymph node metastasis was increased compared to their matched normal tissues. In contrast, levels of ERβ1 and β2 were not correlated with lymph node metastasis, gender, age, tumor size, tumor grade, or pTNM stage[39] (Table 1).
Table 1.
Summary of the association between estrogen receptor isoforms and gastric cancer
| Estrogen receptors | Isoform | Association with gastric cancer |
| ERα | ERα66 | No significant correlation between ERα66 and gastric cancer is found[27] |
| No positive significant difference in both male and female[27,28] | ||
| Incidence higher in poorly differentiated adenocarcinoma[28] | ||
| Association with diffused type gastric cancer is found[31] | ||
| Associated with poor overall survival[31] | ||
| ERα46 | No reported result has been up to date | |
| ERα36 | Expressed highly in gastric cancer[34] | |
| Expression correlated with lymph node metastasis[34] | ||
| Expressed in plasma membrane and cytoplasm of gastric cancer[34] | ||
| ERβ | ERβ1 | Associated with low tumor grades[32] |
| ERβ2 | Presence could have protective effect against invasion[32] | |
| ERβ3 | Absence of ERβ a significant independent prognostic factor for poor OS[15] | |
| ERβ4 | ERβ5 is associated with PTNM stage[39] | |
| ERβ5 |
ER: Estrogen receptor; OS: Overall survival.
MECHANISMS FOR THE FUNCTION OF ERS IN GASTRIC CANCER
Current knowledge on the mechanisms underlying the function of ERs in cancer mainly comes from investigations in breast cancer, which may be extendable to other cancers, including gastric cancer. Estrogens exert their functions via ERs through both genomic and non-genomic pathways[40]. As illustrated in Figure 2, in the genomic pathway, estrogen-bound ERs translocate into the nucleus, bind to estrogen response elements (EREs) in genomic DNA, and regulate the expression of downstream genes. In the non-genomic pathway, ERs interact with some other signaling molecules in several pathways, such as the PI3K/Akt or mitogen activated protein kinase (MAPK) signaling pathway. ERα and ERβ play different roles in both genomic and non-genomic pathways, where ERβ functions as a transdominant inhibitor/competitor of ERα transcriptional activity at sub-saturating hormone levels[41].
Figure 2.
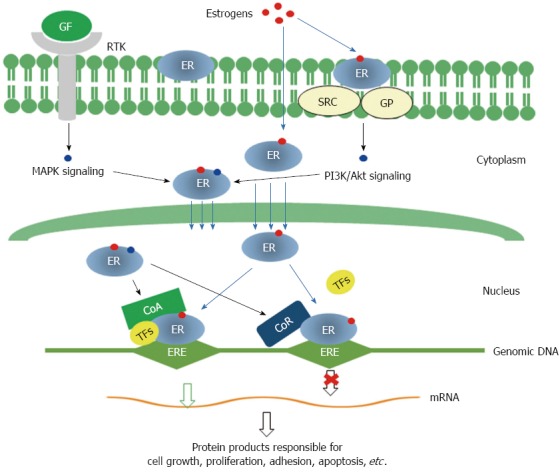
Molecular mechanisms for the functions of estrogen receptors. Genomic pathway: Estrogen binding leads to dimerization of ERs, then ERs translocate into nucleus and interact with transcriptional co-activators and/or co-repressor and bind to genomic DNA at specific sequences known as estrogen response elements (EREs) to activate or repress the transcription of specific genes. Non-genomic signaling pathway: Membrane ERs interact with SRC/G protein and activate PI3K/Akt signaling. Both MAPK signaling initiated by binding of growth factors to receptor tyrosine kinases and PI3K/Akt signaling can modify cytosolic ERs, which may interact with other transcription factors and modulate the transcription of specific genes. GF: Growth factor; RTK: Receptor tyrosine kinase; GP: G proteins; CoA: Transcription co-activator; CoR: Transcription co-receptor; TFs: Transcription factors; MAPK: Mitogen activated protein kinase; PI3K: Phosphoinositide 3 kinase; ERs: Estrogen receptors.
Recently, more investigations were conducted on ERα36-related mechanisms in gastric cancer because of the special characteristics of this newly identified isoform. In established gastric cancer cells, ERα36 protein is mainly expressed on the plasma membrane and in the cytoplasm. Dysregulation of multiple signaling pathways involved in cell proliferation, metastasis, and invasion in relation to ERα36 has been described in gastric cancer[42,43].
ERα36 and glucose regulated protein 94
ERα36 is linked to glucose regulated protein (GRP) 94, as its expression level is positively associated with lymph node metastasis and GRP94 expression levels[34,44,45]. Higher expression of ERα36 in human gastric cancer was involved in the malignant growth of gastric carcinoma cells[34,44]. The Akt signaling pathway is responsible in ERα36-mediated estrogen signaling via GPR-94 in gastric cancer[46]. ERα36 and GRP94 are highly expressed in gastric cancer. With knockdown of ERα36 in gastric cancer SGC-7901 cells, expression of GRP-94 and phosphorylation of Akt (Ser-473-Akt) were reduced significantly. Clinically, GRP94 expression level was significantly correlated with gender, tumor stage, and lymph node metastasis. It is known that estrogen induces the expression of GRPs, which suggests that GRP94 may have some role in gastric carcinogenesis through ERα36-mediated estrogen signaling.
ERα36 and c-Src
C-Src also takes part in ERα36-mediated regulation of gastric cancer cell proliferation by activating the membrane-initiated c-Src signaling pathway. C-Src in breast cancer cells has been reported to serve as a switch through the signal transducer and activator of transcription (STAT) 5/epidermal growth factor receptor (EGFR) pathway in ERα36 mediated biphasic estrogen signaling[47]. It has been reported that ERα36 also interacts physically with Src/Shc/EGFR complex[48]. As seen from these observations, c-Src functions in breast cancer in a similar manner as it does in ERα36-positive gastric cancer[44]. Revealed by the E2-ERα36-c-Src pathway, c-Src transduces signals that are responsible for adhesion, growth, differentiation, and invasion of gastric cancer cells[49]. An important mechanism of c-Src tyrosine kinase activity monitoring is comprised of its phosphorylation status control. C-Src protein has two major phosphorylation sites, Tyr416 and Tyr527. The activity of c-Src is positively regulated when Tyr416 is phosphorylated and it negatively regulated when Tyr416 is dephosphorylated[50,51]. The phosphorylation status of c-Src-Tyr416 and c-Src-Tyr527 depends on the concentration of estrogen and serves to switch on and off non-genomic estrogen signaling[44]. E2-ERα36 regulates phosphorylation of c-Src-Tyr 416 and Tyr 527; as a result, gastric cancer growth is promoted, further indicating that E2-ERα36-c-Src is important for proliferation of gastric cancer cells. C-Src and ERα36 are known to interact in the presence of E2β, while PP2 does not affect this interaction. However, PP2 inhibits the activation of c-Src.
ERα36 and cyclin D1
ERα36 upregulates cyclin D1 (CD1) when activating the c-Src signaling pathway, which leads to the proliferation of gastric cancer cells[34]. In ERα36 up-regulated cells, E2β induces c-Src-Tyr416 phosphorylation[46]. In contrast, E2β was unable to induce c-Src-Tyr527 phosphorylation in cells where ERα36 was knocked down. The level of CDI expression was increased by C-Src-Tyr416 phosphorylation in ERα36 up-regulated SGC7901 cells, and cell proliferation was promoted; while in ERα36-knockdown SGC7901 cells, the opposite occurred. A noteworthy regulatory factor for cell cycle progression is CDI. It mediates the transition from G1 to S, which in turn results in DNA synthesis and cell cycle progression[52]. Various carcinomas were reported to be a result of CDI overexpression, including gastric cancer. A gender difference in methyl-nitro-nitroso-guanidine (MNNG)-induced rat gastric carcinogenesis showed CD1/cdk4 expression[53]. To support these observations further, in tumors in nude mice, the xenograft with up-regulated ERα36 showed a positive correlation between CD1 and ERα36[46].
OTHER ASPECTS OF ERs IN GASTRIC CANCER
ERα is expressed in 20%-30% of human gastric cancers[15]. Epidemiological studies indicate a predominance of gastric cancer in males globally, with the ratio to female as 2:1[54,55]. Antiestrogen and tamoxifen agents have been shown to induce tumor progression and enhance the overall chances of gastric adenocarcinoma[56]. These findings indicate a connection between pathogenesis of gastric cancer and estrogen signaling. Hormone therapy may be a useful strategy for the treatment of gastric cancer in cases of hormone-dependent tumor growth[32].
While the clinicopathological and prognostic relevance of ERs in gastric cancer appears to be significant[16,43], the interaction between the α and β receptors is as yet clinically unclear. Moreover, the positive rate for ER expression in gastric cancer differs from study to study, with ERβ expressed more abundantly than ERα and different patterns in subtypes of gastric cancer. Although some studies showed that aberrant expression of ERα and ERβ mRNAs in tumors is associated with liver metastasis and lymph node metastasis, other have shown that there was no association between expression of ERβ and any clinical variables[57]. Furthermore, the mechanism of carcinogenesis linked to ERβ is unclear, and the use of estrogen for the therapeutic purposes may increase the risk for other cancers (breast or ovarian cancer); the side effects of estrogen are also problematic[15,39,57]. The fractional agonist activity of tamoxifen through ERα in some circumstances can be entirely abolished upon co-expression of ERβ[15]. One possible role of ERβ is to moderate ERα transcriptional activity, and thus the relative expression level of the two isoforms might be a key factor for determining cellular responses to agonists and antagonists. Aromatase expression has been reported in gastric cancer cells recently, and with a short incubation period, gastric cancer can produce estradiol[58].
Notably, the role of estrogen in the stimulation of the growth of gastric cancer cells is associated with the concentration of estrogen[59]. A physiologically low concentration of estrogen was found to stimulate the expression of ERα36 and growth of gastric cancer cells, while high concentrations of estrogen repressed the expression of ERα36 and the growth of gastric cancer cells. This relationship between concentration of estrogen and its function may explain the predominance of gastric cancer in males[60].
PERSPECTIVES
Many studies have been conducted on the expression and association of different isoforms of ERs with gastric cancer, with various conclusions. Some of the inconsistencies may be caused by the variations in these studies, such as in vitro gastric cancer cell models, clinical samples, and assay protocols, with regard to different isoforms of the ERs. Detailed investigations regarding individual isoforms using specific assay protocols (such as specific primer pairs for reverse transcription polymerase chain reaction, antibodies against specific epitopes for each individual isoforms) will no doubt reveal more insight into the role of ERs in gastric cancer. Given the potential of deregulated ERs as diagnostic/prognostic markers or therapeutic targets for gastric cancer, it will be important to identify/confirm the specific roles that each isoform of these ERs (including their tissue-specific ligands) plays under specific conditions in the development and/or progression of gastric cancer, to determine the interactions of these isoforms, and to elucidate the mechanisms at all levels, including molecular, cellular, tissue/organ, and individual. This will provide us a systematic understanding of ERs and provide the basis for developing preventive, diagnostic, and therapeutic approaches with precise targets in ER-related gastric cancer. Furthermore, as new isoforms of ER are being identified and studied in breast cancer, extensive investigation of ER in gastric cancer will surely provide us more knowledge on the development and progression of gastric cancer, and, therefore, will also provide us additional means to combat gastric cancer.
ACKNOWLEDGMENTS
We would like extend our gratitude to Adeeba Mahmood for her help revising English language of our revised manuscript.
Footnotes
Supported by The National Natural Science Foundation of China, No. 30271450, No. 30471955, No. 30672365 and No. 81172516.
Conflict-of-interest statement: The authors declare no conflict of interest for this article.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: October 1, 2015
First decision: November 5, 2015
Article in press: December 14, 2015
P- Reviewer: Aurello P, Kim GH S- Editor: Ma YJ L- Editor: Filipodia E- Editor: Zhang DN
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Lee HR, Kim TH, Choi KC. Functions and physiological roles of two types of estrogen receptors, ERα and ERβ, identified by estrogen receptor knockout mouse. Lab Anim Res. 2012;28:71–76. doi: 10.5625/lar.2012.28.2.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Welboren WJ, Sweep FC, Span PN, Stunnenberg HG. Genomic actions of estrogen receptor alpha: what are the targets and how are they regulated? Endocr Relat Cancer. 2009;16:1073–1089. doi: 10.1677/ERC-09-0086. [DOI] [PubMed] [Google Scholar]
- 4.Lee HR, Hwang KA, Park MA, Yi BR, Jeung EB, Choi KC. Treatment with bisphenol A and methoxychlor results in the growth of human breast cancer cells and alteration of the expression of cell cycle-related genes, cyclin D1 and p21, via an estrogen receptor-dependent signaling pathway. Int J Mol Med. 2012;29:883–890. doi: 10.3892/ijmm.2012.903. [DOI] [PubMed] [Google Scholar]
- 5.Park MA, Hwang KA, Choi KC. Diverse animal models to examine potential role(s) and mechanism of endocrine disrupting chemicals on the tumor progression and prevention: Do they have tumorigenic or anti-tumorigenic property? Lab Anim Res. 2011;27:265–273. doi: 10.5625/lar.2011.27.4.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao C, Dahlman-Wright K, Gustafsson JA. ESR2 (Estrogen Receptor 2 (ER beta) Atlas Genet Cytogenet Oncol Haematol. 2009;13:4. [Google Scholar]
- 7.Wang Z, Zhang X, Shen P, Loggie BW, Chang Y, Deuel TF. Identification, cloning, and expression of human estrogen receptor-alpha36, a novel variant of human estrogen receptor-alpha66. Biochem Biophys Res Commun. 2005;336:1023–1027. doi: 10.1016/j.bbrc.2005.08.226. [DOI] [PubMed] [Google Scholar]
- 8.Flouriot G, Brand H, Denger S, Metivier R, Kos M, Reid G, Sonntag-Buck V, Gannon F. Identification of a new isoform of the human estrogen receptor-alpha (hER-alpha) that is encoded by distinct transcripts and that is able to repress hER-alpha activation function 1. EMBO J. 2000;19:4688–4700. doi: 10.1093/emboj/19.17.4688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Penot G, Le Péron C, Mérot Y, Grimaud-Fanouillère E, Ferrière F, Boujrad N, Kah O, Saligaut C, Ducouret B, Métivier R, et al. The human estrogen receptor-alpha isoform hERalpha46 antagonizes the proliferative influence of hERalpha66 in MCF7 breast cancer cells. Endocrinology. 2005;146:5474–5484. doi: 10.1210/en.2005-0866. [DOI] [PubMed] [Google Scholar]
- 10.Kim KH, Bender JR. Rapid, estrogen receptor-mediated signaling: why is the endothelium so special? Sci STKE. 2005;2005:pe28. doi: 10.1126/stke.2882005pe28. [DOI] [PubMed] [Google Scholar]
- 11.Li L, Haynes MP, Bender JR. Plasma membrane localization and function of the estrogen receptor alpha variant (ER46) in human endothelial cells. Proc Natl Acad Sci USA. 2003;100:4807–4812. doi: 10.1073/pnas.0831079100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moriarty K, Kim KH, Bender JR. Minireview: estrogen receptor-mediated rapid signaling. Endocrinology. 2006;147:5557–5563. doi: 10.1210/en.2006-0729. [DOI] [PubMed] [Google Scholar]
- 13.Xu C, Li J, Lu Y, Jiang Z. Estrogen receptor α and hedgehog signal pathway developmental biology of gastric adenocarcinoma. Hepatogastroenterology. 2012;59:1319–1322. doi: 10.5754/hge11549. [DOI] [PubMed] [Google Scholar]
- 14.Fisher RI, Neifeld JP, Lippman ME. Oestrogen receptors in human malignant melanoma. Lancet. 1976;2:337–339. doi: 10.1016/s0140-6736(76)92592-7. [DOI] [PubMed] [Google Scholar]
- 15.Xu CY, Guo JL, Jiang ZN, Xie SD, Shen JG, Shen JY, Wang LB. Prognostic role of estrogen receptor alpha and estrogen receptor beta in gastric cancer. Ann Surg Oncol. 2010;17:2503–2509. doi: 10.1245/s10434-010-1031-2. [DOI] [PubMed] [Google Scholar]
- 16.Tokunaga A, Nishi K, Matsukura N, Tanaka N, Onda M, Shirota A, Asano G, Hayashi K. Estrogen and progesterone receptors in gastric cancer. Cancer. 1986;57:1376–1379. doi: 10.1002/1097-0142(19860401)57:7<1376::aid-cncr2820570722>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 17.Donnell MS, Meyer GA, Donegan WL. Estrogen-receptor protein in intracranial meningiomas. J Neurosurg. 1979;50:499–502. doi: 10.3171/jns.1979.50.4.0499. [DOI] [PubMed] [Google Scholar]
- 18.Kobayashi S, Mizuno T, Tobioka N, Ichimura H, Samoto T, Tanaka H, Masaoka A, Wakabayashi S, Umemura S, Fukuoka H, et al. Sex steroid receptors in diverse human tumors. Gan. 1982;73:439–445. [PubMed] [Google Scholar]
- 19.Ranelletti FO, Carmignani M, Marchetti P, Natoli C, Iacobelli S. Estrogen binding by neoplastic human thymus cytosol. Eur J Cancer. 1980;16:951–955. doi: 10.1016/0014-2964(80)90334-5. [DOI] [PubMed] [Google Scholar]
- 20.Greenway B, Iqbal MJ, Johnson PJ, Williams R. Oestrogen receptor proteins in malignant and fetal pancreas. Br Med J (Clin Res Ed) 1981;283:751–753. doi: 10.1136/bmj.283.6294.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nemenoff RA, Winn RA. Role of nuclear receptors in lung tumourigenesis. Eur J Cancer. 2005;41:2561–2568. doi: 10.1016/j.ejca.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 22.Shen SS, Smith CL, Hsieh JT, Yu J, Kim IY, Jian W, Sonpavde G, Ayala GE, Younes M, Lerner SP. Expression of estrogen receptors-alpha and -beta in bladder cancer cell lines and human bladder tumor tissue. Cancer. 2006;106:2610–2616. doi: 10.1002/cncr.21945. [DOI] [PubMed] [Google Scholar]
- 23.Hayashi S, Yamaguchi Y. Estrogen signaling pathway and hormonal therapy. Breast Cancer. 2008;15:256–261. doi: 10.1007/s12282-008-0070-z. [DOI] [PubMed] [Google Scholar]
- 24.Wigle DT, Turner MC, Gomes J, Parent ME. Role of hormonal and other factors in human prostate cancer. J Toxicol Environ Health B Crit Rev. 2008;11:242–259. doi: 10.1080/10937400701873548. [DOI] [PubMed] [Google Scholar]
- 25.Hershberger PA, Vasquez AC, Kanterewicz B, Land S, Siegfried JM, Nichols M. Regulation of endogenous gene expression in human non-small cell lung cancer cells by estrogen receptor ligands. Cancer Res. 2005;65:1598–1605. doi: 10.1158/0008-5472.CAN-04-2694. [DOI] [PubMed] [Google Scholar]
- 26.Lewis-Wambi JS, Jordan VC. Treatment of Postmenopausal Breast Cancer with Selective Estrogen Receptor Modulators (SERMs) Breast Dis. 2006;24:93–105. doi: 10.3233/bd-2006-24108. [DOI] [PubMed] [Google Scholar]
- 27.Sica V, Nola E, Contieri E, Bova R, Masucci MT, Medici N, Petrillo A, Weisz A, Molinari AM, Puca GA. Estradiol and progesterone receptors in malignant gastrointestinal tumors. Cancer Res. 1984;44:4670–4674. [PubMed] [Google Scholar]
- 28.Yokozaki H, Takekura N, Takanashi A, Tabuchi J, Haruta R, Tahara E. Estrogen receptors in gastric adenocarcinoma: a retrospective immunohistochemical analysis. Virchows Arch A Pathol Anat Histopathol. 1988;413:297–302. doi: 10.1007/BF00783021. [DOI] [PubMed] [Google Scholar]
- 29.Matsui M, Kojima O, Uehara Y, Takahashi T. Characterization of estrogen receptor in human gastric cancer. Cancer. 1991;68:305–308. doi: 10.1002/1097-0142(19910715)68:2<305::aid-cncr2820680216>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 30.Singh S, Poulsom R, Wright NA, Sheppard MC, Langman MJ. Differential expression of oestrogen receptor and oestrogen inducible genes in gastric mucosa and cancer. Gut. 1997;40:516–520. doi: 10.1136/gut.40.4.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yi JH, Do IG, Jang J, Kim ST, Kim KM, Park SH, Park JO, Park YS, Lim HY, Kang WK, et al. Anti-tumor efficacy of fulvestrant in estrogen receptor positive gastric cancer. Sci Rep. 2014;4:7592. doi: 10.1038/srep07592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ryu WS, Kim JH, Jang YJ, Park SS, Um JW, Park SH, Kim SJ, Mok YJ, Kim CS. Expression of estrogen receptors in gastric cancer and their clinical significance. J Surg Oncol. 2012;106:456–461. doi: 10.1002/jso.23097. [DOI] [PubMed] [Google Scholar]
- 33.Zhou J, Teng R, Xu C, Wang Q, Guo J, Xu C, Li Z, Xie S, Shen J, Wang L. Overexpression of ERα inhibits proliferation and invasion of MKN28 gastric cancer cells by suppressing β-catenin. Oncol Rep. 2013;30:1622–1630. doi: 10.3892/or.2013.2610. [DOI] [PubMed] [Google Scholar]
- 34.Deng H, Huang X, Fan J, Wang L, Xia Q, Yang X, Wang Z, Liu L. A variant of estrogen receptor-alpha, ER-alpha36 is expressed in human gastric cancer and is highly correlated with lymph node metastasis. Oncol Rep. 2010;24:171–176. [PMC free article] [PubMed] [Google Scholar]
- 35.Lazennec G, Bresson D, Lucas A, Chauveau C, Vignon F. ER beta inhibits proliferation and invasion of breast cancer cells. Endocrinology. 2001;142:4120–4130. doi: 10.1210/endo.142.9.8395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weihua Z, Andersson S, Cheng G, Simpson ER, Warner M, Gustafsson JA. Update on estrogen signaling. FEBS Lett. 2003;546:17–24. doi: 10.1016/s0014-5793(03)00436-8. [DOI] [PubMed] [Google Scholar]
- 37.Paruthiyil S, Parmar H, Kerekatte V, Cunha GR, Firestone GL, Leitman DC. Estrogen receptor beta inhibits human breast cancer cell proliferation and tumor formation by causing a G2 cell cycle arrest. Cancer Res. 2004;64:423–428. doi: 10.1158/0008-5472.can-03-2446. [DOI] [PubMed] [Google Scholar]
- 38.Gan L, He J, Zhang X, Zhang YJ, Yu GZ, Chen Y, Pan J, Wang JJ, Wang X. Expression profile and prognostic role of sex hormone receptors in gastric cancer. BMC Cancer. 2012;12:566. doi: 10.1186/1471-2407-12-566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guo JL, Xu CY, Jiang ZN, Dong MJ, Xie SD, Shen JG, Cao J, Wang LB. Estrogen receptor beta variants mRNA expressions in gastric cancer tissues and association with clinicopathologic parameters. Hepatogastroenterology. 2009;57:1584–1588. [PubMed] [Google Scholar]
- 40.Barzi A, Lenz AM, Labonte MJ, Lenz HJ. Molecular pathways: Estrogen pathway in colorectal cancer. Clin Cancer Res. 2013;19:5842–5848. doi: 10.1158/1078-0432.CCR-13-0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kousteni S, Bellido T, Plotkin LI, O’Brien CA, Bodenner DL, Han L, Han K, DiGregorio GB, Katzenellenbogen JA, Katzenellenbogen BS, et al. Nongenotropic, sex-nonspecific signaling through the estrogen or androgen receptors: dissociation from transcriptional activity. Cell. 2001;104:719–730. [PubMed] [Google Scholar]
- 42.Zhao XH, Gu SZ, Liu SX, Pan BR. Expression of estrogen receptor and estrogen receptor messenger RNA in gastric carcinoma tissues. World J Gastroenterol. 2003;9:665–669. doi: 10.3748/wjg.v9.i4.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Matsuyama S, Ohkura Y, Eguchi H, Kobayashi Y, Akagi K, Uchida K, Nakachi K, Gustafsson JA, Hayashi S. Estrogen receptor beta is expressed in human stomach adenocarcinoma. J Cancer Res Clin Oncol. 2002;128:319–324. doi: 10.1007/s00432-002-0336-3. [DOI] [PubMed] [Google Scholar]
- 44.Wang X, Deng H, Zou F, Fu Z, Chen Y, Wang Z, Liu L. ER-α36-mediated gastric cancer cell proliferation via the c-Src pathway. Oncol Lett. 2013;6:329–335. doi: 10.3892/ol.2013.1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fu Z, Deng H, Wang X, Yang X, Wang Z, Liu L. Involvement of ER-α36 in the malignant growth of gastric carcinoma cells is associated with GRP94 overexpression. Histopathology. 2013;63:325–333. doi: 10.1111/his.12171. [DOI] [PubMed] [Google Scholar]
- 46.Fu Z, Zhen H, Zou F, Wang X, Chen Y, Liu L. Involvement of the Akt signaling pathway in ER-α36/GRP94-mediated signaling in gastric cancer. Oncol Lett. 2014;8:2077–2080. doi: 10.3892/ol.2014.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang XT, Ding L, Kang LG, Wang ZY. Involvement of ER-α36, Src, EGFR and STAT5 in the biphasic estrogen signaling of ER-negative breast cancer cells. Oncol Rep. 2012;27:2057–2065. doi: 10.3892/or.2012.1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang XT, Kang LG, Ding L, Vranic S, Gatalica Z, Wang ZY. A positive feedback loop of ER-α36/EGFR promotes malignant growth of ER-negative breast cancer cells. Oncogene. 2011;30:770–780. doi: 10.1038/onc.2010.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Frame MC. Src in cancer: deregulation and consequences for cell behaviour. Biochim Biophys Acta. 2002;1602:114–130. doi: 10.1016/s0304-419x(02)00040-9. [DOI] [PubMed] [Google Scholar]
- 50.Xu W, Harrison SC, Eck MJ. Three-dimensional structure of the tyrosine kinase c-Src. Nature. 1997;385:595–602. doi: 10.1038/385595a0. [DOI] [PubMed] [Google Scholar]
- 51.Xu W, Doshi A, Lei M, Eck MJ, Harrison SC. Crystal structures of c-Src reveal features of its autoinhibitory mechanism. Mol Cell. 1999;3:629–638. doi: 10.1016/s1097-2765(00)80356-1. [DOI] [PubMed] [Google Scholar]
- 52.Sherr CJ. D-type cyclins. Trends Biochem Sci. 1995;20:187–190. doi: 10.1016/s0968-0004(00)89005-2. [DOI] [PubMed] [Google Scholar]
- 53.Motohashi M, Wakui S, Muto T, Suzuki Y, Shirai M, Takahashi H, Hano H. Cyclin D1/cdk4, estrogen receptors α and β, in N-methyl-N’-nitro-N-nitrosoguanidine-induced rat gastric carcinogenesis: immunohistochemical study. J Toxicol Sci. 2011;36:373–378. doi: 10.2131/jts.36.373. [DOI] [PubMed] [Google Scholar]
- 54.Brenner H, Rothenbacher D, Arndt V. Epidemiology of stomach cancer. Methods Mol Biol. 2009;472:467–477. doi: 10.1007/978-1-60327-492-0_23. [DOI] [PubMed] [Google Scholar]
- 55.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 56.Chandanos E, Lindblad M, Rubio CA, Jia C, Warner M, Gustafsson JA, Lagergren J. Tamoxifen exposure in relation to gastric adenocarcinoma development. Eur J Cancer. 2008;44:1007–1014. doi: 10.1016/j.ejca.2008.02.049. [DOI] [PubMed] [Google Scholar]
- 57.Park S, Song J, Joe CO, Shin I. Akt stabilizes estrogen receptor alpha with the concomitant reduction in its transcriptional activity. Cell Signal. 2008;20:1368–1374. doi: 10.1016/j.cellsig.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 58.Maruyama S, Fujimoto N, Asano K, Ito A. Suppression by estrogen receptor beta of AP-1 mediated transactivation through estrogen receptor alpha. J Steroid Biochem Mol Biol. 2001;78:177–184. doi: 10.1016/s0960-0760(01)00083-8. [DOI] [PubMed] [Google Scholar]
- 59.Wang M, Pan JY, Song GR, Chen HB, An LJ, Qu SX. Altered expression of estrogen receptor alpha and beta in advanced gastric adenocarcinoma: correlation with prothymosin alpha and clinicopathological parameters. Eur J Surg Oncol. 2007;33:195–201. doi: 10.1016/j.ejso.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 60.Sipponen P, Correa P. Delayed rise in incidence of gastric cancer in females results in unique sex ratio (M/F) pattern: etiologic hypothesis. Gastric Cancer. 2002;5:213–219. doi: 10.1007/s101200200037. [DOI] [PubMed] [Google Scholar]


