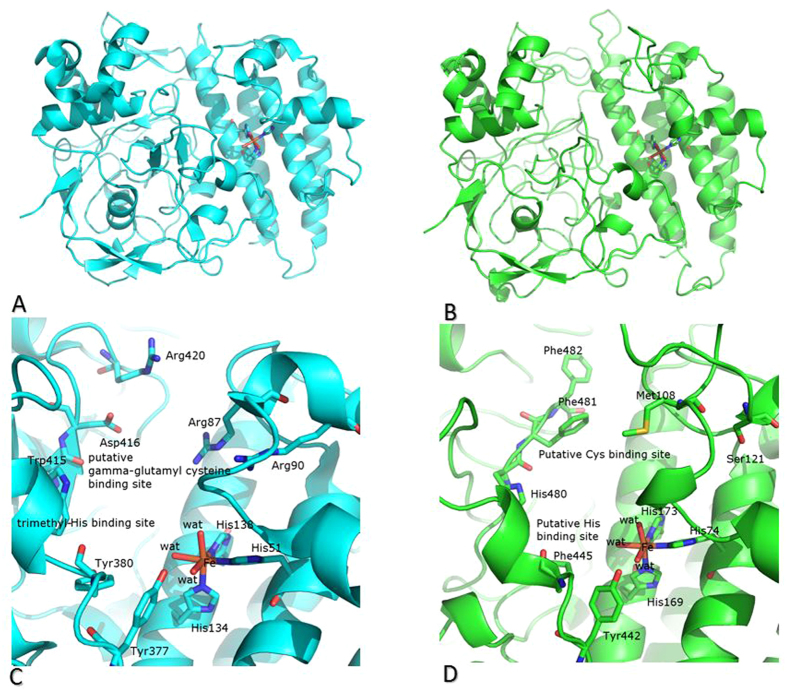
Figure 4. Structural model of PlOvoA.
(A,B) Ribbon representation of the two domains shared by MtEgtB (panel A, in cyan) and PlOvoA (panel B, in green). The iron-binding site and the conserved His residues are also shown as ball and stick. (C,D) Comparison between the active sites of MtEgtB and PlOvoA. MtEgtB (cyan) and PlOvoA (green) catalyze C-S bond formation and sulfoxidation between gamma-glutamyl cysteine and N-alpha-trimethyl histidine or between cysteine and histidine as the central steps in the synthesis of ergothioneine and ovothiol, respectively. Residues involved in the recognition of iron are conserved, whereas those involved in the recognition of N-alpha-trimethyl histidine or important for the binding of gamma-glutamyl cysteine in MtEgtB are not conserved in PlOvoA, according to the different substrates (N-alpha-trimethyl histidine versus His and gamma-glutamyl cysteine versus cysteine) of these enzymes.