Abstract
Shrimps have a widespread distribution across the shelf, slope and seamount regions of the Southern Ocean. Studies of Antarctic organisms have shown that individual species and higher taxa display different degrees of sensitivity and adaptability in response to environmental change. We use species distribution models to predict changes in the geographic range of the deep-sea Antarctic shrimp Nematocarcinus lanceopes under changing climatic conditions from the Last Glacial Maximum to the present and to the year 2100. The present distribution range indicates a pole-ward shift of the shrimp population since the last glaciation. This occurred by colonization of slopes from nearby refugia located around the northern part of Scotia Arc, southern tip of South America, South Georgia, Bouvet Island, southern tip of the Campbell plateau and Kerguelen plateau. By 2100, the shrimp are likely to expand their distribution in east Antarctica but have a continued pole-ward contraction in west Antarctica. The range extension and contraction process followed by the deep-sea shrimp provide a geographic context of how other deep-sea Antarctic species may have survived during the last glaciation and may endure with projected changing climatic conditions in the future.
Keywords: Climate change, Last glacial maximum, Benthos, Range shift, Biogeography, Refugia, Antarctica, Decapod, Species distribution modeling
Introduction
The response of organisms to a changing environment depends on their capacity to cope with the physiological cost imposed by the new conditions and dispersal capacities (Peck, 2004; Peck, 2005; Ingels et al., 2012). Species commonly react to climate change by shifting their latitudinal range (Perry et al., 2005; Parmesan, 2006; Dulvy et al., 2008; Hiddink & Ter Hofstede, 2008; Cheung et al., 2012; Cheung, Watson & Pauly, 2013). Many organisms living in the Antarctic have evolved to survive the combined physiological and ecological constraints of the cold environment (Thatje et al., 2008). During the last glacial maximum (LGM, ca. 19.5–16 ka; Gersonde et al., 2005), Antarctic marine life was challenged by even more extreme environmental conditions with reduced shallow habitat area on the continental shelf and a scarcity of food in the open ocean (i.e., primary production is higher close to coast than open ocean) (Smith & Comiso, 2008). This forced them to take refuge in ice free regions, and then re-colonize their present range (Aronson et al., 2007; Barnes & Conlan, 2007; Thatje et al., 2008). At present, Antarctic ecosystems are experiencing significant environmental changes with the retreat of glaciers and the disintegration of ice shelves due to climate warming suggesting a southward shift of pelagic and benthic communities in the future (Turner et al., 2009). Average global temperature is expected to increase approximately 2 °C in the next 100 years (IPCC Climate Change, 2007). Although, satellite data indicate sea ice extent has not changed markedly over the last 25 years (Bjørgo, Johannessen & Miles, 1997), recent studies suggested ice cover is changing due to climate warming, generally decreasing but increasing in some regions (Polvani & Smith, 2013; Rignot et al., 2013; Simmonds, 2015). The Intergovernmental Panel for Climate Change predicts that a net loss of 25% sea ice cove over the next 100 years would result in a reduced extent of phytoplankton productivity around the Southern Ocean (SO). This may alter food webs through reduced food access coupled with higher metabolic demands due to the warming climate.
The first phylogeographic study of Antarctic shrimps suggested that there was a postglacial expansion of the shelf-inhabiting species Chorismus antarcticus, but not of the deep-water shrimp Nematocarcinus lanceopes (Raupach et al., 2010). Benthic shelf species have been more affected by glaciations than pelagic or deep sea inhabiting species (Janko et al., 2007). However, deep-sea ecosystems may experience abrupt environment changes, such as variation in particulate organic carbon, changing current oscillation pattern etc. (Smith & Kaufmann, 1999; Ruhl & Smith, 2004; Smith et al., 2013). Tropical deep-sea ecosystems fauna may be vulnerable to relatively small changes in temperature (Danovaro, Dell’Anno & Pusceddu, 2004) and so may cold stenothermal polar species (Barnes, Griffiths & Kaiser, 2009). The re-colonization of areas in the Antarctic deep-sea by predators (e.g., litholids) due to climate warming was shown in several past studies (Thatje et al., 2005a; Aronson et al., 2007; Aronson et al., 2009; Griffiths et al., 2013; Kaiser et al., 2013).
Past study methods for single species range-shifts range from spatially explicit mechanistic models (Hill et al., 2001) to climate driven bioclimatic envelope based (Walther, Berger & Sykes, 2005) and correlative species distribution models (SDM) (Peterson & Vieglais, 2001; Pearson et al., 2002; Pearson & Dawson, 2003; Graham et al., 2004; Thuiller et al., 2005; Waltari et al., 2007; Peterson et al., 2011; Bentlage et al., 2013). SDM can provide insights into potential climate warming effects on species even when their physiological limitations are poorly known (Crumpacker, Box & Hardin, 2001; Elith, Kearney & Phillips, 2010). Dambach et al. (2012) used SDM to predict how Antarctic shrimp ranges contracted during the LGM, but did not predict future ranges. In order to understand how shrimps could have survived through past climatic events and how they could respond to future climate change, we ran a SDM using a more comprehensive set of distribution records of the shrimp Nematocarcinus lanceopes and environmental variables representing Past, Present and Future climate conditions. Nematocarcinus lanceopes was selected because it had the most extended distribution records of a deep-sea benthic (Kirkwood, 1984; Arntz et al., 2006; Basher & Costello, 2014). Our findings show how other deep-sea Antarctic species may have survived during the last glaciation and may endure with projected changing climatic conditions in the future.
Materials & Methods
Study area and observation data
Our study area lies in the Southern Ocean between north of the Antarctic Circumpolar Current (ACC) close to 40 °N and the Antarctic coast in the south (Fig. 1). The bathymetry is dominated by deep ocean ridges and a continental shelf break at ca 1,000 m, which is two to four times deeper than the shelf break in other oceanic regions (Knox, 2006). A strong temperature gradient of 4 °C over 0.5° of latitude across the Subtropical front (Sikes et al., 2009) and the ACC distinguishes the Southern Ocean from northern temperate waters. The ACC is the strongest current on Earth and connects the Atlantic, Pacific and Indian ocean basins (Rintoul, Hughes & Olbers, 2001). The ACC creates a physical barrier that has isolated Antarctica for 25 million years (Clarke, Barnes & Hodgson, 2005).
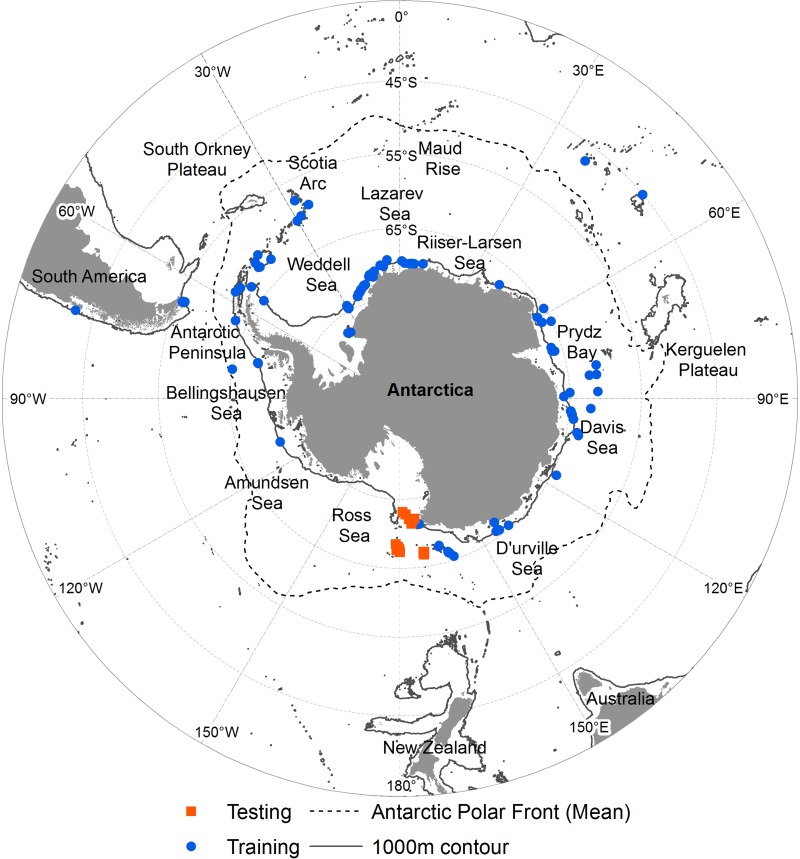
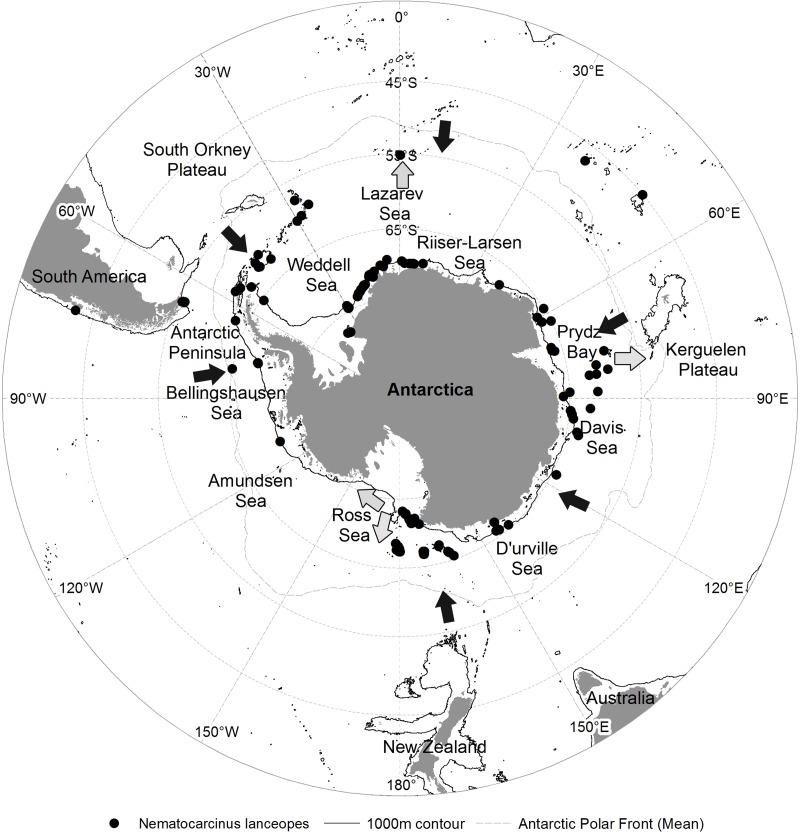
Figure 1. Occurrence of N. lanceopes in the Southern Ocean.
‘Circles’ represent the locations used for model training and ‘squares’ represent the locations used for independent model testing.
A total of 87 N. lanceopes observation records were obtained from the Ocean Biogeographic Information System (OBIS, 2011), the SCAR-Marine Biodiversity Information Network (De Broyer & Danis, 2011), and literature (Fig. 1 and Table S1). An additional 30 records from a recent cruise in the Ross Sea were used for model validation (Basher, Bowden & Costello, 2014a). All records were filtered to remove apparent geographic errors (i.e., coordinates plotting on land or in different regions) before combining them into a single dataset for model training or validation using ArcGIS (ESRI, 2011). All of the data used have been submitted to SCAR MARBIN for open-access online publication following publication of Basher & Costello (2014). The data will thus also become accessible through OBIS and GBIF.
Environmental data
Environmental data were obtained from the Global Marine Environment Datasets (GMED) (Basher, Costello & Bowden, 2014b), namely depth, temperature, salinity, ice cover and primary productivity. The variables were derived from remotely sensed and in-situ measured datasets, and had a spatial resolution (pixel size) of 5 arc-min or ca. 9 km near the equator. As shrimps are predominantly benthic, we used environmental variables reflecting environment conditions near seabed (e.g., in Present and Future models). Unfortunately no seabed environmental layers were available for paleo (i.e., Past) conditions, thus we selected surface layers as a proxy of the seabed conditions. The data set for the past (i.e., LGM) comprised of depth (Depth, m), ice thickness (IceT, m), surface salinity (sSal, ppt) and sea surface temperature (SST, °C). Bottom temperature and salinity layers were only available for Present and Future layers. The dataset for Present and Future conditions was comprised of depth (Depth, m), sea bottom salinity (bSal, ppt), sea bottom temperature (SBT, °C), ice cover (IceC, 0–1%) and primary productivity (Prod, mgC m−2/day).We used the Institut Pierre Simon Laplace (IPSL; http://icmc.ipsl.fr/) Future climate A2 scenario for the environmental data of the year 2100. Our scenario selection was limited to A2 as the deep-sea data layers in other climate scenarios were not available and generating them for this specific study by compiling raw data was beyond the scope of the study. The Depth in Future scenario was considered the same as Present depth since Future predictions of sea level change were currently not available. All variables were derived from mean annual average of in-situ or satellite data . (see Basher, Costello & Bowden, 2014b for details about all layers). High correlations between environmental predictors may not only show spurious results as well as negatively affect SDM performance and its transferability through space and time (Heikkinen et al., 2006; Bigg et al., 2008; Jiménez-Valverde et al., 2009; Liu, White & Newell, 2009; Dormann et al., 2013). None of the environmental variables used in our models showed strong correlations (R2 > 0.7) when tested for pair-wise correlations using Pearson’s correlation.
Model building
MaxEnt 3.3.3e (Phillips, Anderson & Schapire, 2006) was used to model the current distribution of N. lanceopes and to project Past and Future distribution ranges. The program uses a machine learning algorithm following the principles of maximum entropy (Jaynes, 1982). Reviews comparing up to 16 models and of >200 taxa found that machine-learning methods including MaxEnt consistently outperformed traditional linear methods (Elith et al., 2006; Meißner et al., 2014) and that presence-only models were preferable because limited sampling can increase the prevalence of false absences within a dataset. MaxEnt starts with a uniform distribution during the modelling process, and successively fits the model to the data (occurrence records and environmental variables). MaxEnt repeatedly tests the predictive capability of the model and improves by iteratively permuting and varying the input variables and features thereof. This is measured in the log likelihood or “model gain”, which illustrates the discrepancy between the model identified distribution and the uniform distribution (Elith et al., 2011). MaxEnt thus specifies the relative suitability of the environment (interpreted as the potential geographic distribution) of the study organism.
MaxEnt models were generated using 10 bootstrap replicate runs with 100,000 random background points. The average of the 10 predictions across all replicates was used for further analysis. We excluded duplicate records that fell within individual pixels of background environment layers on each dataset using ‘Remove duplicate presence records’ feature in the MaxEnt software. The occurrence records were also split into 75% for training and 25% for testing for bootstrap replications. We set the maximum iterations to 1,000, to facilitate model convergence. As suggested by Phillips & Dudik (2008) the default regularization (i.e., smoothing) value was used because it results in better performance of evaluation data for presence-only datasets. We minimized unreliable extrapolation into areas with environmental conditions that were not encountered during model training using the ‘fade by clamping’ option of the software. Any predicted areas having the prediction value below the Minimum Presence Threshold (MPT) were considered unsuitable for the species. Models were projected onto ‘Past’ and ‘Future’ environmental datasets at the end of the iteration phase in two separate instances. As the final procedure, in ArcGIS 10 (ESRI, 2011) we calculated the species range shift maps by subtracting Past SDM raster from the Present SDM raster and then the Present SDM raster from the Future raster to get the Present and Future change maps respectively.
Model evaluation
The logistic model output format gives a predicted suitability value ranging from 0 (unsuitable) to 1 (optimal) (Phillips & Dudik, 2008). The final output raster was classified into four classes based on the range of predicted suitability value: HS (High Suitability, 0.75-Maximum); MS (Medium Suitability, 0.5–0.75); LS (Low Suitability, MPT-0.5) and NS (Not suitable, Values below MPT). These classified raster files were used to interpret the suitability of N. lanceopes environment in the Southern Ocean. MaxEnt allows for model evaluation by the Area Under the Receiver Operating Characteristic Curve (AUC) (Phillips, Dudík & Schapire, 2004). AUC is a threshold-independent measurement of model discrimination. An AUC value of 0.5 indicates model predictions are not better than random and AUC > 0.9 indicates high performance (Peterson et al., 2011). We used a random data split approach to evaluate model performance using a bootstrap procedure with an evaluation dataset (25% of the entire Present species distribution records). We used percent variable contribution and jack-knife procedures in the software to investigate the relative importance of different environmental predictors. The jack-knife procedure produces a model by using variables in isolation to examine how well the result fits the known model gain (for both training and test data). Response curves were used to evaluate the relationships between environmental variables and the predicted presence probability of N. lanceopes. Confidence maps were generated using the ratio of the standard deviation of the MaxEnt prediction maps to the mean environment suitability. Using an independent dataset is the optimal method for evaluating model performance (Phillips & Dudik, 2008; Kumar & Stohlgren, 2009). Probability of occurrence values, which ranged from 0 to 1, where 0 meant no probability of presence and 1 meant highest probability of presence at that particular location, were extracted from the average of all bootstrap models on each data set using the “Extract Values to Point” function of Spatial Analyst in ArcGIS. We evaluated model accuracy with the independent dataset by seeing how successfully the model predicted the species’ potential distribution outside its given sampling locations.
Results
Predicted distributions
All the SDM had a high predictive power based on the values of AUC > 0.95 (AUC ± SD, Past 0.950 ± 0.01; Future 0.968 ± 0.008). The minimum presence threshold (MPT) values were 0.012 and 0.015 for Past and Future models respectively. The relative importance of the environmental variables to the SDM showed that depth had the highest explanatory power 61–79% for both Past (Table 1 and Fig. 2) and Future (Table 1 and Fig. 4) climate conditions. The second and third most important variables were temperature (26% for Past) and ice cover (9% for Future) (Table 1) (Fig. S2). Independent records used to validate model were all plotted into areas having prediction value above MPT suggests high predictive performance of all the models.
Table 1. Summary of MaxEnt results from the Past and Future models.
The high values of ‘Contribution’ and ‘Permutation Importance’ indicated that Depth, Temperature and Ice Cover were the main predictors regulating the distribution of N. lanceopes in the Southern Ocean. ‘Without predictor’ values indicated model performance when models were developed with all other variables excluding that individual predictor and ‘Only with predictor’ indicated models developed with only that predictor.
| Model Summary | Past | Future | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Training samples | 54 | 54 | |||||||
| Test samples | 18 | 18 | |||||||
| Training gain | 2.17 | 2.51 | |||||||
| Training AUC ± SD | 0.950 ± 0.01 | 0.968 ± 0.008 | |||||||
| Test AUC ± SD | 0.903 ± 0.03 | 0.956 ± 0.02 | |||||||
| Minimum presence threshold | 0.012 | 0.015 | |||||||
| Predictors influence | |||||||||
| Depth | SST | sSal | IceT | Depth | SBT | bSal | Prod | IceC | |
| Contribution (%) | 79.57 | 18.42 | 1.02 | 0.99 | 61.03 | 5.27 | 0.07 | 2.51 | 31.12 |
| Permutation importance | 71.91 | 26.43 | 1.49 | 0.16 | 88.29 | 0.74 | 0.02 | 2.1 | 8.84 |
| Without predictor | |||||||||
| Training gain | 0.53 | 1.89 | 2.15 | 2.15 | 1.28 | 2.44 | 2.51 | 2.43 | 2.22 |
| Test gain | −0.56 | 0.68 | 0.4 | 1.74 | 1.43 | 2.53 | 2.63 | 2.6 | 2.28 |
| AUC | 0.699 | 0.91 | 0.904 | 0.922 | 0.902 | 0.954 | 0.956 | 0.962 | 0.944 |
| Only with predictor | |||||||||
| Training Gain | 1.75 | 0.47 | 0.14 | 0.04 | 1.76 | 0.92 | 0 | 0.13 | 0.76 |
| Test gain | 1.77 | −0.02 | 0.13 | −0.03 | 1.78 | 1.06 | 0 | 0.09 | 0.86 |
| AUC | 0.92 | 0.704 | 0.63 | 0.493 | 0.928 | 0.866 | 0.528 | 0.658 | 0.845 |
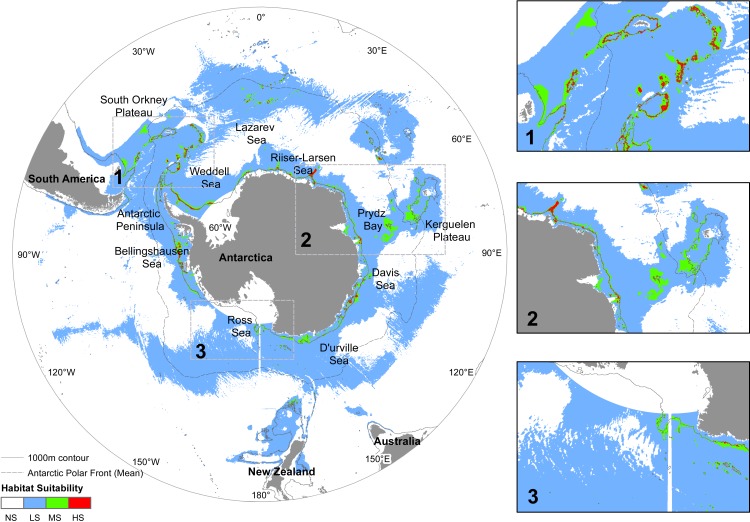
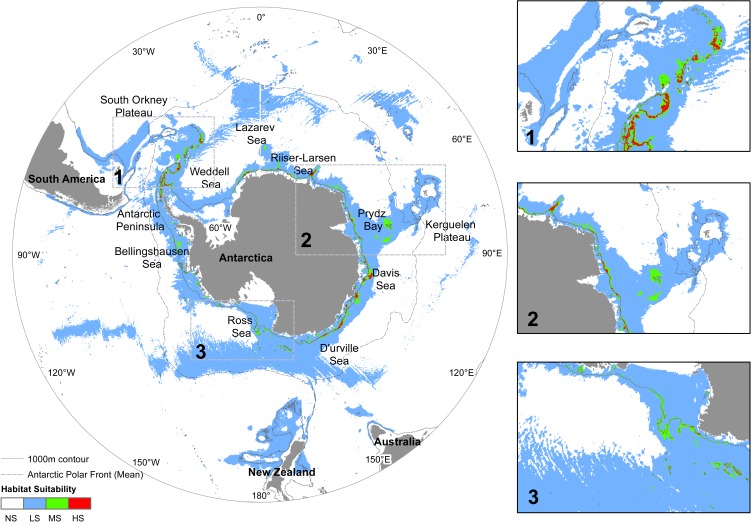
Figure 2. Predicted distribution of N. lanceopes during the Last Glacial Maximum.
Environment suitability: HS, High suitability (red); MS, Medium suitability (green); LS, Low suitability (sky); NS, Not suitable (white). Close up maps of: 1, Scotia Arc and Antarctic Peninsula; 2, Prydz Bay and Kerguelen Plateau; 3, Ross Sea and Amundsen Sea showed in the close up boxes on the right.
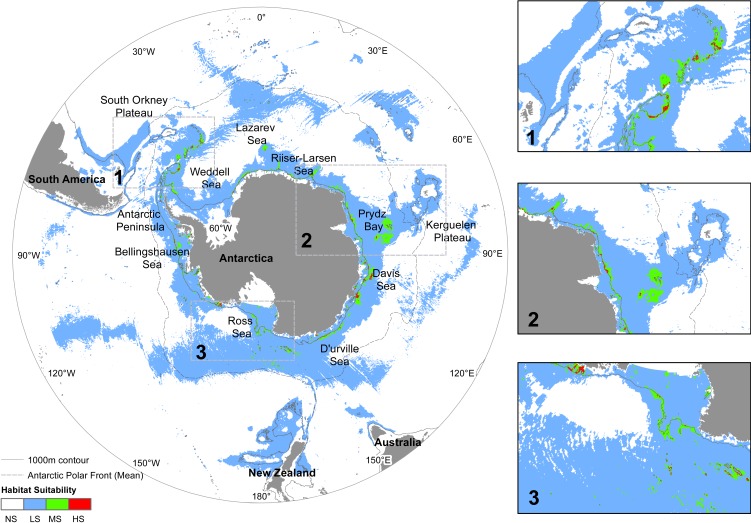
Figure 4. Predicted distribution of N. lanceopes in the Future (year 2100).
Environment suitability: HS, High suitability (red); MS, Medium suitability (green); LS, Low suitability (sky); NS, Not suitable (white). Close up maps of: 1, Scotia Arc and Antarctic Peninsula; 2, Prydz Bay and Kerguelen Plateau; 3, Ross Sea and Amundsen Sea showed in the close up boxes on the right.
Past (LGM) distribution
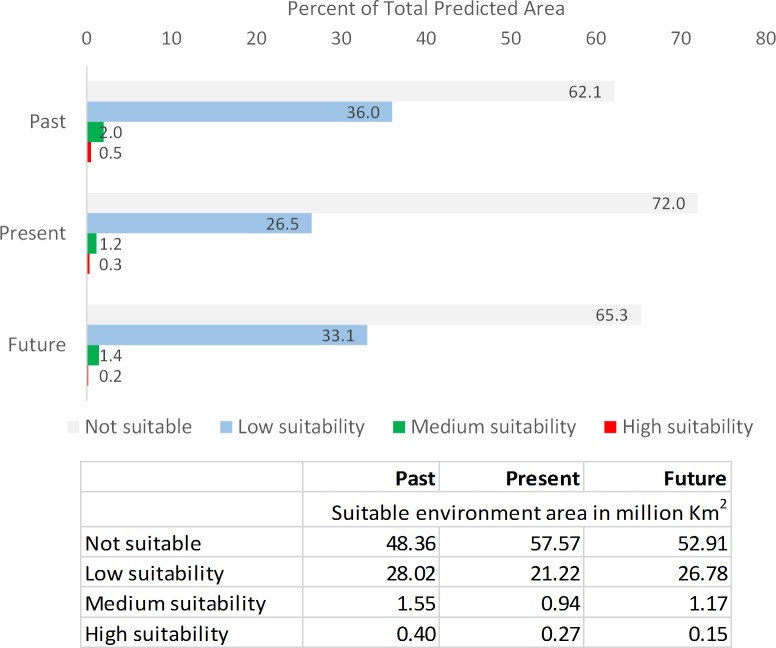
The predicted distribution for the Past indicated that N. lanceopes would have been widely distributed in the Sub-Antarctic regions near the Scotia Arc (South Georgia, South Orkney, South Sandwich Islands), Kerguelen Plateau, Mawson Sea, D’Urville Sea and in the Bellingshausen Sea (Fig. 2). The maximum predicted environment suitability value was 0.875 (Table 1). The high confidence in prediction indicated optimum model performance in identifying potential glacial refugia (i.e., areas with persistent population over time) (Fig. S4). Model predicted about 30 million-km2 area (i.e., sum of LS, MS and HS) suitable for N. lanceopes environment during LGM. More than half of the areas (62%) were identified as ‘not suitable’ for N. lanceopes. The areas having low, medium and high environment suitability were 36%, 2% and 0.5% respectively (Fig. 5).
Figure 5. Variation in the area identified as suitable environment for N. lanceopes in the MaxEnt model predictions.
Environment suitability in the graph: Not suitable (grey); Low suitability (sky); Medium suitability (green); High suitability (red).
Present distribution
The predicted Present distribution covered the current known distribution range of the species. The highest predicted suitability was in areas near the Mawson Sea, Kerguelen Plateau, Ross Sea slope, Davos Sea, Prydz Bay, South Orkney Islands, Bellingshausen Sea and at Gunnerus Ridge in between Riiser-Larsen and the Cosmonaut Sea (Fig. 3). The Present distribution range suggested a pole-ward shift of N. lanceopes after the LGM by colonization of previously unoccupied slope areas. All of the independent validation records occurred in areas having medium to high probability of predicted N. lanceopes distribution (Fig. 1). The model predicted a contraction of suitable environment in the present since the LGM day (22.5 million km2), because more areas (72%) identified as ‘not suitable’ environments in compared to the Past (Fig. 5).
Figure 3. Predicted distribution of N. lanceopes at Present.
Environment suitability: HS, High suitability (red); MS, Medium suitability (green); LS, Low suitability (sky); NS, Not suitable (white). Close up maps of: 1, Scotia Arc and Antarctic Peninsula; 2, Prydz Bay and Kerguelen Plateau; 3, Ross Sea and Amundsen Sea showed in the close up boxes on the right.
Future distribution
The SDM under the predicted Future (2100) climate conditions showed further contraction of N. lanceopes distribution, although there was an increase in suitable areas in the deeper slope regions (Figs. 4 and 7). The potential range predicted by the model showed range expansion into the deeper sections of the eastern Ross Sea shelf, areas between Amundsen Sea and Ross Sea, slopes of D’Urville Sea, Prydz Bay, Maud Rise, bathyal (i.e., seabed) regions of Mawson Sea, Prydz Bay and to the Aurora Canyon near the eastern tip of the Antarctic Peninsula (Fig. 4). The maximum predicted environment suitability value was 0.94 (Table 1). However, the predicted areas with ‘high suitability’ values continued to decrease (0.18%) in the Future, while and and environment areas with ‘low suitability’ and ‘medium suitability’ increased slightly (33%) and (1.45%) respectively (Fig. 5). The model predicted an overall expanded distribution (28 million km2) in Future, with all of the potential expansion areas adjacent to existing N. lanceopes populations. Thus, these areas would be likely to be colonised (Figs. 4 and 7).
Figure 7. Predicted range contraction and expansion direction of N. lanceopes populations in the Southern Ocean based on the model predictions of Past, Present and Future environment conditions in relation to present population locations.
Contraction (black arrow), expansion (grey arrow) and present population locations (black dots).
Effect of climate change
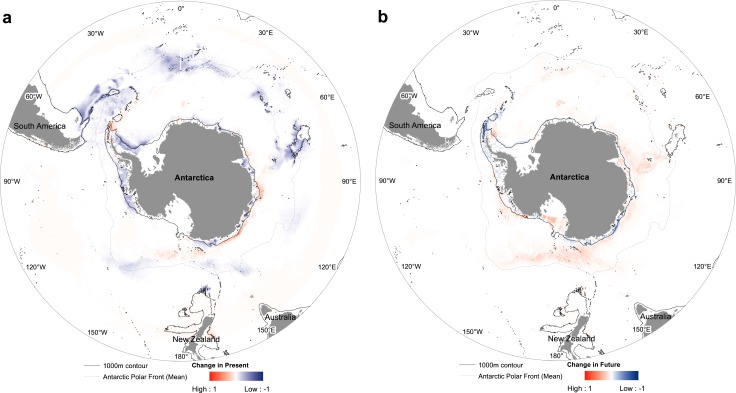
The results indicated a pole-ward shift between the predicted distribution of Past (LGM) and Present day, and Present to Future (year 2100). The highly suitable LGM areas located in the Sub-Antarctic (South Sandwich, South Orkney Islands, and South Georgia), Bouvet Island, Western Weddell Sea and the Kerguelen plateau regions became contracted into smaller areas now. The model also suggested colonization of slope areas of east Antarctica (D’Urville Sea, Davis Sea, and Ross Sea) and the tip of the Antarctic Peninsula (Fig. 6A).
Figure 6. Nematocarcinus lanceopes range loss and gain from (A) Past last glacial maximum period to Present day, (B) from Present to Future.
Areas in ‘red’ indicate gained range and areas in ‘blue’ lost range.
The predicted distribution for the Future followed the previous trend of pole-ward range shift of N. lanceopes populations. However, a range expansion was predicted into newer regions of deeper slope areas near the Scott Seamount in the Ross Sea, Marie Byrd Seamounts in the Amundsen Sea, Aurora Canyon in the eastern tip of the Antarctic Peninsula, and Maud Rise north of the Lazarev Sea (Fig. 6B). Nevertheless, the predicted change in area was not the same for all Antarctic regions. It contracted more in the western Antarctic (Antarctic Peninsula) and expanded more in the Eastern Antarctic regions, i.e. Bellingshausen Sea and eastern Ross Sea. The eastern Ross Sea, which currently covered by ice all year round, was predicted to have more open ocean (i.e., ice free) areas in the Future (Future ice cover in Figs. S1 and S3C) which would facilitate N. lanceopes colonization from the nearby slope areas in the west (Fig. 7).
Discussion
Nematocarcinus lanceopes is the most widely distributed deep-sea shrimp in the Southern Ocean (Arntz & Gorny, 1991; Gutt, Gorny & Arntz, 1991; Arntz et al., 1999; Guzmán & Quiroga, 2005; Lovrich et al., 2005; Thatje, Bacardit & Arntz, 2005b; Donnelly, Sutton & Torres, 2006; Basher & Costello, 2014). We found that the geographic distribution of N. lanceopes was most influenced by depth, ice cover and temperature; supporting previous studies (Dambach et al., 2012; Basher, Bowden & Costello, 2014a). As found by Barnes, Griffiths & Kaiser (2009) for Antarctic benthic gastropods and bivalves, N. lanceopes in our models showed a contraction and expansion of distribution following the variation of ice cover (Fig. 7). This suggests that our findings are more widely applicable to benthic species in Antarctica.
Temporal prediction of species range extension
Species distribution models can predict the direction of species range contractions or expansions (Araújo et al., 2005) but projections beyond the temporal range of a training dataset (i.e., distribution in future dates) require a cautious interpretation to avoid potential pitfalls. The AUC value tends to increase when the selected background area is larger than the species observed presence area (Phillips & Dudik, 2008; Merow, Smith & Silander, 2013). Although using AUC as the only method of model validation has its own caveats (Jiménez-Valverde & Lobo, 2007; Lobo, Jimenez-Valverde & Real, 2008; Pineda & Lobo, 2009), it has been used widely in SDM studies for past and future climate conditions (Lobo, Jimenez-Valverde & Hortal, 2010; Varela, Lobo & Hortal, 2011; Dambach et al., 2012; Weinmann et al., 2013). In addition to AUC, we used model confidence maps and found a consistency in predictive power of the models to characterize the distribution of the species in different temporal resolution, and identified regions that contained less variation in predictions (Fig. S4). All of the three confidence maps have high confidence values for our predictions; indicating that all of the modelled predictions were likely to reflect actual distributions range for the species (Phillips, Anderson & Schapire, 2006; Anderson & Gonzalez Jr, 2011; Davies & Guinotte, 2011).
Many shelf and slope inhabiting Antarctic fauna have an extended bathymetric range (Brey et al., 1996; Basher & Costello, 2014) akin to deep-sea organisms in other oceans (Clarke, 2003). This suggests that Antarctic fauna may represent an evolutionary history of movement in and out of deep water, driven by glacial cycles (Aronson et al., 2007; Fraser et al., 2012). During the LGM, turbidity and currents due to ice scour were likely to have affected the survival of fauna on the continental slope around Antarctica (Thatje, Hillenbrand & Larter, 2005c; Thatje et al., 2008). For most benthic taxa, survival was possible in deep-sea refugia close to the Antarctic continent due to open ocean polynya that supplied food from primary production at the surface (Thatje et al., 2008). Our Past model also suggested LGM refugia around the northern part of the Scotia Arc, southern tip of South America, South Georgia, Bouvet Island, southern tip of the Campbell Plateau and Kerguelen Plateau (Fig. 2). The refugia near Campbell Plateau and Bouvet Island were not identified in a previous study by Dambach et al. (2012) due to their more limited data. Molecular studies on Antarctic isopods, amphipods, and bivalves have indicated similar re-colonization events in nearby shelf and slope from glacial refugia in several taxa since the LGM (Rogers, 2007; Newman et al., 2009). The shrimp refugia found in this study complement these molecular studies and provide a geographic context of how species ranges adjust to environmental change by moving up and down the continental slope and on and off the continental shelf (Fig. 7).
The Antarctic Peninsula warmed 3.7 ± 1.6 °C over the last century (Vaughan et al., 2003; Clarke et al., 2007), while areas in Halley and Amundsen-Scott at the South Pole cooled (Turner et al., 2005). Sea ice cover in the Amundsen Sea reduced over the last three decades and the trend seems set to continue in Future (Fig. S1 and Rignot et al., 2013). Food availability in the deep sea is dependent upon the surface productivity and vertical supply of organic matter from the upper ocean (Smith & Comiso, 2008). Thus, an increase in food availability in the deep sea generally triggers a significant meiofaunal response (Gooday, 2002) resulting in an increase of overall benthic biomass (Levin et al., 2001). As sea ice melts, new environment areas will become available in the shelf and slope for re-colonization which will be supported with increased projected chlorophyll-a production areas (Shepherd, Wingham & Rignot, 2004; Whitehouse et al., 2008; Gerringa et al., 2012).
The overall suitable environmental areas for benthic shrimps in the Antarctic Peninsula shrinks in the Present and Future models compared to the Past model (Figs. 2, 3 and 7). In contrast, the Amundsen Sea has increased suitable area from the Past to the Future models (Figs. 3, 4 and 7). Other regions where environment suitability is projected to increase in the Future include the deeper slopes of the Kerguelen Plateau and the eastern Ross Sea. The Kerguelen Plateau is one of the major linear shelves near Antarctica and has a strong temperature gradient compared to the Antarctic Peninsula and Victoria Land areas. This makes this area likely to experience thermally driven range shifts of Antarctic fauna (Barnes, Griffiths & Kaiser, 2009). With projected warming of the temperature and decreased ice coverage around these regions in the next 100 years, N. lanceopes is likely to expand in these regions (Fig. 4).
Conclusion
We modelled the potential distribution of the deep-sea shrimp Nematocarcinus lanceopes in the Southern Ocean. Results indicated a contraction of suitable environment from the Sub-Antarctic regions and pole-ward expansion on the continental slopes from the LGM to Present, and Present to year 2100. However, an expansion of areas with a suitable environment in the future was predicted for eastern Antarctica but contraction in the western Antarctic. Further research should examine how typical these changes will be of other Southern Ocean species and how benthic communities and food webs will change.
Supplemental Information
Scales range from high (red), to low (blue).
Black indicates high confidence or less variation in predicted performance among all replicates.
Funding Statement
The research was funded by the New Zealand Government under the New Zealand International Polar Year-Census of Antarctic Marine Life Project (Phase 1: So001IPY; Phase 2: IPY2007-01) and University of Auckland Doctoral Scholarship. We gratefully acknowledge project governance by the Ministry of Primary Industries Science Team and the Ocean Survey 20/20 CAML Advisory Group (Land Information New Zealand, Ministry of Primary Industries, Antarctica New Zealand, Ministry of Foreign Affairs and Trade, and National Institute of Water and Atmosphere Ltd). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
Mark J. Costello is an Academic Editor for PeerJ.
Author Contributions
Zeenatul Basher conceived and designed the experiments, performed the experiments, analyzed the data, contributed reagents/materials/analysis tools, wrote the paper, prepared figures and/or tables, reviewed drafts of the paper.
Mark J. Costello conceived and designed the experiments, contributed reagents/materials/analysis tools, wrote the paper, reviewed drafts of the paper.
Data Availability
The following information was supplied regarding data availability:
The research in this article did not generate any raw data and all datasets used for the research are publicly available from different sources which were mentioned in Supplemental Information.
References
- Anderson & Gonzalez Jr (2011).Anderson RP, Gonzalez Jr I. Species-specific tuning increases robustness to sampling bias in models of species distributions: an implementation with Maxent. Ecological Modelling. 2011;222:2796–2811. doi: 10.1016/j.ecolmodel.2011.04.011. [DOI] [Google Scholar]
- Araújo et al. (2005).Araújo MB, Pearson RG, Thuiller W, Erhard M. Validation of species–climate impact models under climate change. Global Change Biology. 2005;11:1504–1513. doi: 10.1111/j.1365-2486.2005.01000.x. [DOI] [Google Scholar]
- Arntz & Gorny (1991).Arntz WE, Gorny M. Shrimp (Decapoda, Natantia) occurrence and distribution in the Eastern Weddell Sea, Antarctica. Polar Biology. 1991;11:169–177. [Google Scholar]
- Arntz et al. (1999).Arntz WE, Gorny M, Soto R, Lardies MA, Retamal M, Wehrtmann IS. Species composition and distribution of decapod crustaceans in the waters off Patagonia and Tierra del Fuego, South America. Scientia Marina. 1999;63:303–314. [Google Scholar]
- Arntz et al. (2006).Arntz WE, Thatje S, Linse K, Avila C, Ballesteros M, Barnes DKA, Cope T, Cristobo FJ, De Broyer C, Gutt J, Isla E, Lopez-Gonzalez P, Montiel A, Munilla T, Espla AAR, Raupach M, Rauschert M, Rodriguez E, Teixido N. Missing link in the Southern Ocean: sampling the marine benthic fauna of remote Bouvet Island. Polar Biology. 2006;29:83–96. doi: 10.1007/s00300-005-0047-8. [DOI] [Google Scholar]
- Aronson et al. (2009).Aronson RB, Moody RM, Ivany LC, Blake DB, Werner JE, Glass A. Climate change and trophic response of the Antarctic bottom fauna. PLoS ONE. 2009;4:e1713. doi: 10.1371/journal.pone.0004385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronson et al. (2007).Aronson RB, Thatje S, Clarke A, Peck LS, Blake DB, Wilga CD, Seibel BA. Climate change and invasibility of the antarctic benthos. Annual Review of Ecology, Evolution, and Systematics. 2007;38:129–154. doi: 10.1146/annurev.ecolsys.38.091206.095525. [DOI] [Google Scholar]
- Barnes & Conlan (2007).Barnes DKA, Conlan KE. Disturbance, colonization and development of Antarctic benthic communities. Philosophical Transactions of the Royal Society B-Biological Sciences. 2007;362:11–38. doi: 10.1098/rstb.2006.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes, Griffiths & Kaiser (2009).Barnes DKA, Griffiths HJ, Kaiser S. Geographic range shift responses to climate change by Antarctic benthos: where we should look. Marine Ecology-Progress Series. 2009;393:13–26. doi: 10.3354/meps08246. [DOI] [Google Scholar]
- Basher, Bowden & Costello (2014a).Basher Z, Bowden DA, Costello MJ. Diversity and distribution of deep-sea shrimps in the Ross Sea region of Antarctica. PLoS ONE. 2014a;9:e1713. doi: 10.1371/journal.pone.0103195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basher & Costello (2014).Basher Z, Costello MJ. Chapter 5.22. Shrimps (Crustacea: Decapoda) In: De Broyer CKP, Griffiths HJ, Raymond B, Udekem d’Acoz Cd’, Van de Putte AP, Danis B, David B, Grant S, Gutt J, Held C, Hosie G, Huettmann F, Post A, Ropert-Coudert Y, editors. Biogeographic Atlas of the Southern Ocean. Cambridge: Scientific Committee on Antarctic Research; 2014. pp. 190–194. [Google Scholar]
- Basher, Costello & Bowden (2014b).Basher Z, Costello MJ, Bowden DA. Global marine environment dataset (GMED) (Version 1.0) 2014b Available at http://gmed.auckland.ac.nz (accessed 17 March 2014)
- Bentlage et al. (2013).Bentlage B, Peterson AT, Barve N, Cartwright P. Plumbing the depths: extending ecological niche modelling and species distribution modelling in three dimensions. Global Ecology and Biogeography. 2013;22:952–961. doi: 10.1111/geb.12049. [DOI] [Google Scholar]
- Bigg et al. (2008).Bigg GR, Cunningham CW, Ottersen G, Pogson GH, Wadley MR, Williamson P. Ice-age survival of Atlantic cod: agreement between palaeoecology models and genetics. Proceedings of the Royal Society B: Biological Sciences. 2008;275:163–172. doi: 10.1098/rspb.2007.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjørgo, Johannessen & Miles (1997).Bjørgo E, Johannessen OM, Miles MW. Analysis of merged SMMR-SSMI time series of Arctic and Antarctic sea ice parameters 1978–1995. Geophysical Research Letters. 1997;24:413–416. doi: 10.1029/96GL04021. [DOI] [Google Scholar]
- Brey et al. (1996).Brey T, Dahm C, Gorny M, Klages M, Stiller M, Arntz WE. Do Antarctic benthic invertebrates show an extended level of eurybathy? Antarctic Science. 1996;8:3–6. doi: 10.1017/S0954102096000028. [DOI] [Google Scholar]
- Cheung et al. (2012).Cheung WWL, Meeuwig JJ, Feng M, Harvey E, Lam VWY, Langlois T, Slawinski D, Sun CJ, Pauly D. Climate-change induced tropicalisation of marine communities in Western Australia. Marine and Freshwater Research. 2012;63:415–427. doi: 10.1071/MF11205. [DOI] [Google Scholar]
- Cheung, Watson & Pauly (2013).Cheung WWL, Watson R, Pauly D. Signature of ocean warming in global fisheries catch. Nature. 2013;497:365–368. doi: 10.1038/Nature12156. [DOI] [PubMed] [Google Scholar]
- Clarke (2003).Clarke A. The polar deep seas. In: Tyler P, editor. Ecosystems of the world. Amsterdam: Elsevier; 2003. pp. 239–260. [Google Scholar]
- Clarke, Barnes & Hodgson (2005).Clarke A, Barnes DKA, Hodgson DA. How isolated is Antarctica? Trends in Ecology & Evolution. 2005;20:1–3. doi: 10.1016/j.tree.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Clarke et al. (2007).Clarke A, Murphy EJ, Meredith MP, King JC, Peck LS, Barnes DKA, Smith RC. Climate change and the marine ecosystem of the western Antarctic Peninsula. Philosophical Transactions of the Royal Society B: Biological Sciences. 2007;362:149–166. doi: 10.1098/rstb.2006.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crumpacker, Box & Hardin (2001).Crumpacker DW, Box EO, Hardin ED. Implications of climatic warming for conservation of native trees and shrubs in Florida. Conservation Biology. 2001;15:1008–1020. doi: 10.1046/j.1523-1739.2001.0150041008.x. [DOI] [Google Scholar]
- Dambach et al. (2012).Dambach J, Thatje S, Rödder D, Basher Z, Raupach MJ. Effects of Late-Cenozoic glaciation on habitat availability in Antarctic benthic shrimps (Crustacea: Decapoda: Caridea) PLoS ONE. 2012;7:e1713. doi: 10.1371/journal.pone.0046283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danovaro, Dell’Anno & Pusceddu (2004).Danovaro R, Dell’Anno A, Pusceddu A. Biodiversity response to climate change in a warm deep sea. Ecology Letters. 2004;7:821–828. doi: 10.1111/j.1461-0248.2004.00634.x. [DOI] [Google Scholar]
- Davies & Guinotte (2011).Davies AJ, Guinotte JM. Global habitat suitability for framework-forming cold-water corals. PLoS ONE. 2011;6:e1713. doi: 10.1371/journal.pone.0018483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Broyer & Danis (2011).De Broyer C, Danis B, editors. SCAR-MarBIN: The Antarctic marine biodiversity information network. 2011. Available at http://wwwscarmarbinbe/ (accessed 07 July 2011) [Google Scholar]
- Donnelly, Sutton & Torres (2006).Donnelly J, Sutton TT, Torres JJ. Distribution and abundance of micronekton and macrozooplankton in the NW Weddell Sea: relation to a spring ice-edge bloom. Polar Biology. 2006;29:280–293. doi: 10.1007/s00300-005-0051-z. [DOI] [Google Scholar]
- Dormann et al. (2013).Dormann CF, Elith J, Bacher S, Buchmann C, Carl G, Carré G, Marquéz JRG, Gruber B, Lafourcade B, Leitão PJ, Münkemüller T, McClean C, Osborne PE, Reineking B, Schröder B, Skidmore AK, Zurell D, Lautenbach S. Collinearity: a review of methods to deal with it and a simulation study evaluating their performance. Ecography. 2013;36:027–046. doi: 10.1111/j.1600-0587.2012.07348.x. [DOI] [Google Scholar]
- Dulvy et al. (2008).Dulvy NK, Rogers SI, Jennings S, Stelzenmuller V, Dye SR, Skjoldal HR. Climate change and deepening of the North Sea fish assemblage: a biotic indicator of warming seas. Journal of Applied Ecology. 2008;45:1029–1039. doi: 10.1111/j.1365-2664.2008.01488.x. [DOI] [Google Scholar]
- Elith et al. (2006).Elith J, Graham CH, Anderson RP, Dudík M, Ferrier S, Guisan A, Hijmans RJ, Huettmann F, Leathwick JR, Lehmann A, Li J, Lohmann LG, Loiselle BA, Manion G, Moritz C, Nakamura M, Nakazawa Y, McC M, Overton J, Townsend Peterson A, Phillips SJ, Richardson K, Scachetti-Pereira R, Schapire RE, Soberón J, Williams S, Wisz MS, Zimmermann NE. Novel methods improve prediction of species’ distributions from occurrence data. Ecography. 2006;29:129–151. doi: 10.1111/j.2006.0906-7590.04596.x. [DOI] [Google Scholar]
- Elith, Kearney & Phillips (2010).Elith J, Kearney M, Phillips S. The art of modelling range-shifting species. Methods in Ecology and Evolution. 2010;1:330–342. doi: 10.1111/j.2041-210X.2010.00036.x. [DOI] [Google Scholar]
- Elith et al. (2011).Elith J, Phillips SJ, Hastie T, Dudík M, Chee YE, Yates CJ. A statistical explanation of MaxEnt for ecologists. Diversity and Distributions. 2011;17:43–57. doi: 10.1111/j.1472-4642.2010.00725.x. [DOI] [Google Scholar]
- ESRI (2011).ESRI . ArcGIS desktop: release 10. Redlands: Environmental Systems Research Institute; 2011. [Google Scholar]
- Fraser et al. (2012).Fraser CI, Nikula R, Ruzzante DE, Waters JM. Poleward bound: biological impacts of Southern Hemisphere glaciation. Trends in Ecology & Evolution. 2012;27:462–471. doi: 10.1016/j.tree.2012.04.011. [DOI] [PubMed] [Google Scholar]
- Gerringa et al. (2012).Gerringa LJ, Alderkamp A-C, Laan P, Thuroczy C-E, De Baar HJ, Mills MM, Van Dijken GL, Haren HV, Arrigo KR. Iron from melting glaciers fuels the phytoplankton blooms in Amundsen Sea (Southern Ocean): iron biogeochemistry. Deep Sea Research Part II: Topical Studies in Oceanography. 2012;71:16–31. doi: 10.1016/j.dsr2.2012.03.007. [DOI] [Google Scholar]
- Gersonde et al. (2005).Gersonde R, Crosta X, Abelmann A, Armand L. Sea-surface temperature and sea ice distribution of the Southern Ocean at the EPILOG Last Glacial Maximum—a circum-Antarctic view based on siliceous microfossil records. Quaternary Science Reviews. 2005;24:869–896. doi: 10.1016/j.quascirev.2004.07.015. [DOI] [Google Scholar]
- Gooday (2002).Gooday AJ. Biological responses to seasonally varying fluxes of organic matter to the ocean floor: a review. Journal of Oceanography. 2002;58:305–332. doi: 10.1023/A:1015865826379. [DOI] [Google Scholar]
- Graham et al. (2004).Graham CH, Ferrier S, Huettman F, Moritz C, Peterson AT. New developments in museum-based informatics and applications in biodiversity analysis. Trends in Ecology & Evolution. 2004;19:497–503. doi: 10.1016/j.tree.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Griffiths et al. (2013).Griffiths HJ, Whittle RJ, Roberts SJ, Belchier M, Linse K. Antarctic crabs: invasion or endurance? PLoS ONE. 2013;8:e1713. doi: 10.1371/journal.pone.0066981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutt, Gorny & Arntz (1991).Gutt J, Gorny M, Arntz W. Spatial-distribution of Antarctic shrimps (Crustacea, Decapoda) by underwater photography. Antarctic Science. 1991;3:363–369. [Google Scholar]
- Guzmán & Quiroga (2005).Guzmán G, Quiroga E. New records of shrimps (Decapoda: Caridea and Dendrobranchiata) in deep waters of Chile. Gayana (Concepcin) 2005;69:285–290. doi: 10.4067/S0717-6538200500020000. [DOI] [Google Scholar]
- Heikkinen et al. (2006).Heikkinen RK, Luoto M, Araújo MB, Virkkala R, Thuiller W, Sykes MT. Methods and uncertainties in bioclimatic envelope modelling under climate change. Progress in Physical Geography. 2006;30:751–777. doi: 10.1177/0309133306071957. [DOI] [Google Scholar]
- Hiddink & Ter Hofstede (2008).Hiddink J, Ter Hofstede R. Climate induced increases in species richness of marine fishes. Global Change Biology. 2008;14:453–460. doi: 10.1111/j.1365-2486.2007.01518.x. [DOI] [Google Scholar]
- Hill et al. (2001).Hill JK, Collingham YC, Thomas CD, Blakeley DS, Fox R, Moss D, Huntley B. Impacts of landscape structure on butterfly range expansion. Ecology Letters. 2001;4:313–321. doi: 10.1046/j.1461-0248.2001.00222.x. [DOI] [Google Scholar]
- Ingels et al. (2012).Ingels J, Vanreusel A, Brandt A, Catarino AI, David B, De Ridder C, Dubois P, Gooday AJ, Martin P, Pasotti F, Robert H. Possible effects of global environmental changes on Antarctic benthos: a synthesis across five major taxa. Ecology and Evolution. 2012;2:453–485. doi: 10.1002/ece3.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IPCC Climate Change (2007).IPCC Climate Change . Working Group II Report “Impacts, Adaptation and Vulnerability”. In: Parry OFC ML, Palutikof JP, Van der Linden PJ, Hanson CE, editors. Contribution of working group II to the fourth assessment report of the intergovernmental panel on climate change, 2007. Cambridge: Cambridge University Press; 2007. p. 976. [Google Scholar]
- Janko et al. (2007).Janko K, Lecointre G, DeVries A, Couloux A, Cruaud C, Marshall C. Did glacial advances during the Pleistocene influence differently the demographic histories of benthic and pelagic Antarctic shelf fishes?–Inferences from intraspecific mitochondrial and nuclear DNA sequence diversity. BMC Evolutionary Biology. 2007;7:220. doi: 10.1186/1471-2148-7-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaynes (1982).Jaynes ET. On the rationale of maximum-entropy methods. Proceedings of the IEEE. 1982;70:939–952. doi: 10.1109/PROC.1982.12425. [DOI] [Google Scholar]
- Jiménez-Valverde & Lobo (2007).Jiménez-Valverde A, Lobo JM. Threshold criteria for conversion of probability of species presence to either–or presence–absence. Acta Oecologica. 2007;31:361–369. doi: 10.1016/j.actao.2007.02.001. [DOI] [Google Scholar]
- Jiménez-Valverde et al. (2009).Jiménez-Valverde A, Nakazawa Y, Lira-Noriega A, Peterson AT. Environmental correlation structure and ecological niche model projections. Biodiversity Informatics. 2009;6:28–35. doi: 10.17161/bi.v6i1.1634. [DOI] [Google Scholar]
- Kaiser et al. (2013).Kaiser S, Brandão S, Brix S, Barnes DA, Bowden D, Ingels J, Leese F, Schiaparelli S, Arango C, Badhe R, Bax N, Blazewicz-Paszkowycz M, Brandt A, Brenke N, Catarino A, David B, Ridder C, Dubois P, Ellingsen K, Glover A, Griffiths H, Gutt J, Halanych K, Havermans C, Held C, Janussen D, Lörz A-N, Pearce D, Pierrat B, Riehl T, Rose A, Sands C, Soler-Membrives A, Schüller M, Strugnell J, Vanreusel A, Veit-Köhler G, Wilson N, Yasuhara M. Patterns, processes and vulnerability of Southern Ocean benthos: a decadal leap in knowledge and understanding. Marine Biology. 2013;160:2295–2317. doi: 10.1007/s00227-013-2232-6. [DOI] [Google Scholar]
- Kirkwood (1984).Kirkwood JM. ANARE Res Notes. Kingston: Information Services Section, Antarctic Division, Dept. of Science and Technology; 1984. A guide to the Decapoda of the Southern Ocean; pp. 1–47. [Google Scholar]
- Knox (2006).Knox GA. Biology of the Southern Ocean. 2nd edition. CRC Press; 2006. The Southern Ocean; pp. 1–16. [Google Scholar]
- Kumar & Stohlgren (2009).Kumar S, Stohlgren TJ. Maxent modeling for predicting suitable habitat for threatened and endangered tree Canacomyrica monticola in New Caledonia. Journal of Ecology and The Natural Environment. 2009;1:94–98. [Google Scholar]
- Levin et al. (2001).Levin LA, Etter RJ, Rex MA, Gooday AJ, Smith CR, Pineda J, Stuart CT, Hessler RR, Pawson D. Environmental influences on regional deep-sea species diversity. Annual Review of Ecology and Systematics. 2001;32:51–93. [Google Scholar]
- Liu, White & Newell (2009).Liu C, White M, Newell G. Assessing the accuracy of species distribution models more thoroughly. 18th world Imacs congress and Modsim09 international congress on modelling and simulation; 2009. pp. 4234–4240. [Google Scholar]
- Lobo, Jimenez-Valverde & Hortal (2010).Lobo JM, Jimenez-Valverde A, Hortal J. The uncertain nature of absences and their importance in species distribution modelling. Ecography. 2010;33:103–114. doi: 10.1111/j.1600-0587.2009.06039.x. [DOI] [Google Scholar]
- Lobo, Jimenez-Valverde & Real (2008).Lobo JM, Jimenez-Valverde A, Real R. AUC: a misleading measure of the performance of predictive distribution models. Global Ecology and Biogeography. 2008;17:145–151. doi: 10.1111/j.1466-8238.2007.00358.x. [DOI] [Google Scholar]
- Lovrich et al. (2005).Lovrich GA, Romero MC, Tapella F, Thatje S. Distribution, reproductive and energetic conditions of decapod crustaceans along the Scotia Arc (Southern Ocean) Scientia Marina. 2005;69:183–193. [Google Scholar]
- Meißner et al. (2014).Meißner K, Fiorentino D, Schnurr S, Martinez Arbizu P, Huettmann F, Holst S, Brix S, Svavarsson J. Distribution of benthic marine invertebrates at northern latitudes—an evaluation applying multi-algorithm species distribution models. Journal of Sea Research. 2014;85:241–254. doi: 10.1016/j.seares.2013.05.007. [DOI] [Google Scholar]
- Merow, Smith & Silander (2013).Merow C, Smith MJ, Silander JA. A practical guide to MaxEnt for modeling species’ distributions: what it does, and why inputs and settings matter. Ecography. 2013;36:1058–1069. doi: 10.1111/j.1600-0587.2013.07872.x. [DOI] [Google Scholar]
- Newman et al. (2009).Newman L, Convey P, Gibson J, Linse K. Antarctic paleobiology: glacial refugia and constraints on past icesheet reconstructions. PAGES News. 2009;17:22–24. [Google Scholar]
- OBIS (2011).OBIS Data from the Ocean Biogeographic Information System. Intergovernmental Oceanographic Commission of UNESCO. 2011. Available at http://wwwiobisorg (accessed 5 July 2011)
- Parmesan (2006).Parmesan C. Ecological and evolutionary responses to recent climate change. Annual Review of Ecology, Evolution, and Systematics. 2006;37:637–669. doi: 10.1146/annurev.ecolsys.37.091305.110100. [DOI] [Google Scholar]
- Pearson & Dawson (2003).Pearson RG, Dawson TP. Predicting the impacts of climate change on the distribution of species: are bioclimate envelope models useful? Global Ecology and Biogeography. 2003;12:361–371. doi: 10.1046/j.1466-822X.2003.00042.x. [DOI] [Google Scholar]
- Pearson et al. (2002).Pearson RG, Dawson TP, Berry PM, Harrison PA. SPECIES: a spatial evaluation of climate impact on the envelope of species. Ecological Modelling. 2002;154:289–300. doi: 10.1016/S0304-3800(02)00056-X. [DOI] [Google Scholar]
- Peck (2005).Peck L. Prospects for surviving climate change in Antarctic aquatic species. Frontiers in Zoology. 2005;2:9. doi: 10.1186/1742-9994-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peck (2004).Peck LS. Physiological flexibility: the key to success and survival for Antarctic fairy shrimps in highly fluctuating extreme environments. Freshwater Biology. 2004;49:1195–1205. doi: 10.1111/j.1365-2427.2004.01264.x. [DOI] [Google Scholar]
- Perry et al. (2005).Perry AL, Low PJ, Ellis JR, Reynolds JD. Climate change and distribution shifts in marine fishes. Science. 2005;308:1912–1915. doi: 10.1126/science.1111322. [DOI] [PubMed] [Google Scholar]
- Peterson et al. (2011).Peterson AT, Soberón J, Pearson RG, Anderson RP, Martínez-Meyer E, Nakamura M, Araújo MB. Ecological niches and geographic distributions. Princeton: Princeton University Press; 2011. [Google Scholar]
- Peterson & Vieglais (2001).Peterson AT, Vieglais DA. Predicting species invasions using ecological niche modeling: new approaches from bioinformatics attack a pressing problem. Bioscience. 2001;51:363–371. doi: 10.1641/0006-3568(2001)051[0363:PSIUEN]2.0.CO;2. [DOI] [Google Scholar]
- Phillips, Anderson & Schapire (2006).Phillips SJ, Anderson RP, Schapire RE. Maximum entropy modeling of species geographic distributions. Ecological Modelling. 2006;190:231–259. doi: 10.1016/j.ecolmodel.2005.03.026. [DOI] [Google Scholar]
- Phillips & Dudik (2008).Phillips SJ, Dudik M. Modeling of species distributions with Maxent: new extensions and a comprehensive evaluation. Ecography. 2008;31:161–175. doi: 10.1111/j.0906-7590.2008.5203.x. [DOI] [Google Scholar]
- Phillips, Dudík & Schapire (2004).Phillips SJ, Dudík M, Schapire RE. A maximum entropy approach to species distribution modeling. In: Greiner R, Schuurmans D, editors. Twenty-first international conference on machine learning, ICML 2004; Banff, Alta. 2004. pp. 655–662. [Google Scholar]
- Pineda & Lobo (2009).Pineda E, Lobo JM. Assessing the accuracy of species distribution models to predict amphibian species richness patterns. Journal of Animal Ecology. 2009;78:182–190. doi: 10.1111/j.1365-2656.2008.01471.x. [DOI] [PubMed] [Google Scholar]
- Polvani & Smith (2013).Polvani LM, Smith KL. Can natural variability explain observed Antarctic sea ice trends? New modeling evidence from CMIP5. Geophysical Research Letters. 2013;40:3195–3199. doi: 10.1002/grl.50578. [DOI] [Google Scholar]
- Raupach et al. (2010).Raupach MJ, Thatje S, Dambach J, Rehm P, Misof B, Leese F. Genetic homogeneity and circum-Antarctic distribution of two benthic shrimp species of the Southern Ocean, Chorismus antarcticus and Nematocarcinus lanceopes. Marine Biology. 2010;157:1783–1797. doi: 10.1007/s00227-010-1451-3. [DOI] [Google Scholar]
- Rignot et al. (2013).Rignot E, Jacobs S, Mouginot J, Scheuchl B. Ice-shelf melting around Antarctica. Science. 2013;341:266–270. doi: 10.1126/science.1235798. [DOI] [PubMed] [Google Scholar]
- Rintoul, Hughes & Olbers (2001).Rintoul S, Hughes C, Olbers D. The Antarctic circumpolar current system. In: Siedler G, Church J, Gould J, editors. Ocean circulation and climate. New York: Academic Press; 2001. pp. 271–302. [Google Scholar]
- Rogers (2007).Rogers AD. Evolution and biodiversity of Antarctic organisms: a molecular perspective. Philosophical Transactions of the Royal Society B: Biological Sciences. 2007;362:2191–2214. doi: 10.1098/rstb.2006.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruhl & Smith (2004).Ruhl HA, Smith KL. Shifts in deep-sea community structure linked to climate and food supply. Science. 2004;305:513–515. doi: 10.1126/science.1099759. [DOI] [PubMed] [Google Scholar]
- Shepherd, Wingham & Rignot (2004).Shepherd A, Wingham D, Rignot E. Warm ocean is eroding West Antarctic ice sheet. Geophysical Research Letters. 2004;31 doi: 10.1029/2004GL021106. L23402. [DOI] [Google Scholar]
- Sikes et al. (2009).Sikes EL, Howard WR, Samson CR, Mahan TS, Robertson LG, Volkman JK. Southern Ocean seasonal temperature and Subtropical Front movement on the South Tasman Rise in the late Quaternary. Paleoceanography. 2009;24 doi: 10.1029/2008pa001659. PA2201. [DOI] [Google Scholar]
- Simmonds (2015).Simmonds I. Comparing and contrasting the behaviour of Arctic and Antarctic sea ice over the 35 year period 1979–2013. Annals of Glaciology. 2015;56:18–28. doi: 10.3189/2015AoG69A909. [DOI] [Google Scholar]
- Smith & Kaufmann (1999).Smith KL, Kaufmann RS. Long-term discrepancy between food supply and demand in the deep eastern North Pacific. Science. 1999;284:1174–1177. doi: 10.1126/science.284.5417.1174. [DOI] [PubMed] [Google Scholar]
- Smith et al. (2013).Smith KL, Ruhl HA, Kahru M, Huffard CL, Sherman AD. Deep ocean communities impacted by changing climate over 24 y in the abyssal northeast Pacific Ocean. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:19838–19841. doi: 10.1073/pnas.1315447110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith & Comiso (2008).Smith WO, Comiso JC. Influence of sea ice on primary production in the Southern Ocean: a satellite perspective. Journal of Geophysical Research-Oceans. 2008;113 doi: 10.1029/2007jc004251. C05S93. [DOI] [Google Scholar]
- Thatje et al. (2005a).Thatje S, Anger K, Calcagno JA, Lovrich GA, Portner HO, Arntz WE. Challenging the cold: crabs reconquer the Antarctic. Ecology. 2005a;86:619–625. doi: 10.1890/04-0620. [DOI] [Google Scholar]
- Thatje, Bacardit & Arntz (2005b).Thatje S, Bacardit R, Arntz W. Larvae of the deep-sea Nematocarcinidae (Crustacea : Decapoda : Caridea) from the southern ocean. Polar Biology. 2005b;28:290–302. doi: 10.1007/s00300-004-0687-0. [DOI] [Google Scholar]
- Thatje, Hillenbrand & Larter (2005c).Thatje S, Hillenbrand CD, Larter R. On the origin of Antarctic marine benthic community structure. Trends in Ecology & Evolution. 2005c;20:534–540. doi: 10.1016/j.tree.2005.07.010. [DOI] [PubMed] [Google Scholar]
- Thatje et al. (2008).Thatje S, Hillenbrand CD, Mackensen A, Larter R. Life hung by a thread: endurance of Antarctic fauna in glacial periods. Ecology. 2008;89:682–692. doi: 10.1890/07-0498.1. [DOI] [PubMed] [Google Scholar]
- Thuiller et al. (2005).Thuiller W, Richardson DM, Pyssek P, Midgley GF, Hughes GO, Rouget M. Niche-based modelling as a tool for predicting the risk of alien plant invasions at a global scale. Global Change Biology. 2005;11:2234–2250. doi: 10.1111/j.1365-2486.2005.001018.x. [DOI] [PubMed] [Google Scholar]
- Turner et al. (2009).Turner J, Bindschadler R, Convey P, Di Prisco G, Fahrbach E, Gutt J, Hodgson D, Mayewski P, Summerhayes C. Antarctic climate change and the environment. Cambridge: Scientific Committeee for Antarctic Research; 2009. [Google Scholar]
- Turner et al. (2005).Turner J, Colwell SR, Marshall GJ, Lachlan-Cope TA, Carleton AM, Jones PD, Lagun V, Reid PA, Iagovkina S. Antarctic climate change during the last 50 years. International Journal of Climatology. 2005;25:279–294. doi: 10.1002/joc.1130. [DOI] [Google Scholar]
- Varela, Lobo & Hortal (2011).Varela S, Lobo JM, Hortal J. Using species distribution models in paleobiogeography: A matter of data, predictors and concepts. Palaeogeography, Palaeoclimatology, Palaeoecology. 2011;310:451–463. doi: 10.1016/j.palaeo.2011.07.021. [DOI] [Google Scholar]
- Vaughan et al. (2003).Vaughan DG, Marshall GJ, Connolley WM, Parkinson C, Mulvaney R, Hodgson DA, King JC, Pudsey CJ, Turner J. Recent rapid regional climate warming on the Antarctic Peninsula. Climatic Change. 2003;60:243–274. doi: 10.1023/A:1026021217991. [DOI] [Google Scholar]
- Waltari et al. (2007).Waltari E, Hijmans RJ, Peterson AT, Nyári ÁS, Perkins SL, Guralnick RP. Locating pleistocene refugia: comparing phylogeographic and ecological niche model predictions. PLoS ONE. 2007;2:e1713. doi: 10.1371/journal.pone.0000563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther, Berger & Sykes (2005).Walther G-R, Berger S, Sykes MT. An ecological ‘footprint’of climate change. Proceedings of the Royal Society B: Biological Sciences. 2005;272:1427–1432. doi: 10.1098/rspb.2005.3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinmann et al. (2013).Weinmann AE, Rödder D, Lötters S, Langer MR. Traveling through time: the past, present and future biogeographic range of the invasive foraminifera Amphistegina spp. in the Mediterranean Sea. Marine Micropaleontology. 2013;105:30–39. doi: 10.1016/j.marmicro.2013.10.002. [DOI] [Google Scholar]
- Whitehouse et al. (2008).Whitehouse MJ, Meredith MP, Rothery P, Atkinson A, Ward P, Korb RE. Rapid warming of the ocean around South Georgia, Southern Ocean, during the 20th century: forcings, characteristics and implications for lower trophic levels. Deep-Sea Research Part I-Oceanographic Research Papers. 2008;55:1218–1228. doi: 10.1016/j.dsr.2008.06.002. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Scales range from high (red), to low (blue).
Black indicates high confidence or less variation in predicted performance among all replicates.
Data Availability Statement
The following information was supplied regarding data availability:
The research in this article did not generate any raw data and all datasets used for the research are publicly available from different sources which were mentioned in Supplemental Information.