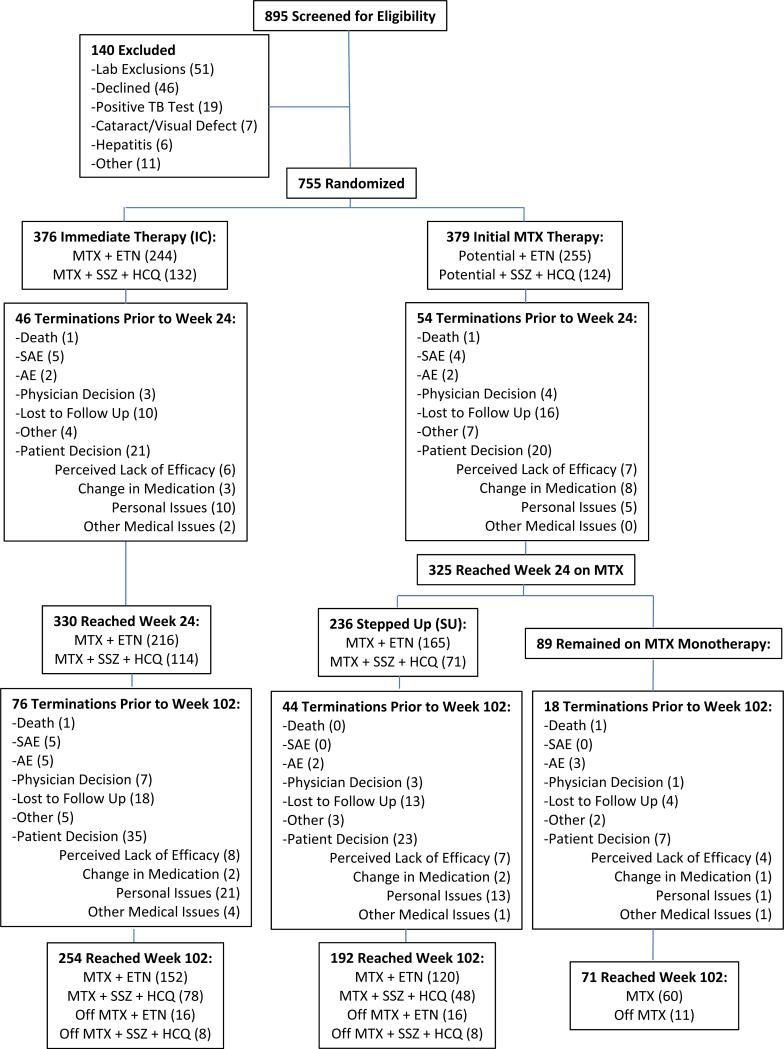
Figure 1.
Flow diagram showing the distribution of the study subjects from initial assessment to analysis of the study data. Details are given according to the Consolidated Standards of Reporting Trials (CONSORT) statement for reporting randomized controlled trials. Although 89 participants had a Disease Activity Score in 28 joints using the erythrocyte sedimentation rate of 3.2 at week 24, only 81 continued in the study past week 24. Off medication means the subject was off any/all active study drugs. TB, tuberculosis; MTX, methotrexate; ETN, etanercept; SSZ, sulfasalazine; HCQ, hydroxychloroquine; SAE, serious adverse event; AE, adverse event; MM, MTX monotherapy.