Abstract
MX belongs to a family of type I interferon (IFN)-stimulated genes, and the MX protein has antiviral activity. MX has at least two isoforms, known as MX1 and MX2, in mammals. Moreover, bovine MX1 has been found to have alternative splice variants—namely, MX1-a and MX1B. In ruminants, IFN-τ—a type I IFN—is temporarily produced from the conceptus before implantation and induces MX expression in the endometrium. However, the expression dynamics of MX after implantation are not clear. In the present study, we investigated the expression of MX1-a, MX1B and MX2 in the endometrium and placenta before and after implantation along with the expression of IFN-α, type I receptors (IFNAR1 and IFNAR2) and interferon regulatory factors (IRF3 and IRF9). Pregnant uterine samples were divided into five groups according to pregnancy days 14–18, 25–40, 50–70, 80–100, and 130–150. Tissue samples were collected from the intercaruncular endometrium (IC), caruncular endometrium (C) and fetal placenta (P). Although all the MX expressions were significantly higher in the IC and C at days 14–18, presumably caused by embryo-secreted IFN-τ stimulation, their expressions were also detectable in the IC, C and P after implantation. Furthermore, IFN-α expression was significantly higher in the IC. RT-PCR indicated IFNAR1, IFNAR2, IRF3 and IRF9 mRNA in all the tissues during pregnancy. These results suggest that all the MX genes are affected by the type I IFN pathway during pregnancy and are involved in an immune response to protect the mother and fetus.
Keywords: Cow, MX, Uterus
In ruminants such as cows and sheep, a type I interferon (IFN)—IFN-τ—is secreted from the trophoblast before implantation [1]. IFN-τ is known as a pregnancy recognition signal that inhibits oxytocin receptor expression and the production of PGF2α and maintains the corpus luteum and pregnancy [2]. Several IFN-stimulated genes (ISGs) are expressed in uterine tissue via IFN-τ stimulation [3,4,5]. The Myxovirus resistance (MX) genes are ISGs, and their levels of mRNA and protein increase in the endometrium at the time of implantation [6,7,8,9,10,11]. The in vitro expression of MX1 and MX2 mRNA in bovine epithelial cells is stimulated by IFN-τ [10, 12]. This IFN-τ signaling pathway is called the janus kinase (JAK)-signal transducer and activator of transcription (STAT) signaling pathway, and it is activated via a transmembrane receptor composed of the IFNAR1 and IFNAR2 subunits [2,3,4, 13]. IFN-τ production occurs only at preimplantation, and it is not clear whether MX mRNA is induced by the IFN signaling pathway during pregnancy.
On the other hand, IFN is typically produced in innate immune responses in mammals. When bacteria and viruses infect animals, pattern recognition receptors (PRRs), such as Toll-like receptors (TLRs) and RIG-I-like receptors, recognize pathogen-associated molecular patterns and trigger the type I IFN signaling pathway [14]. IFN-α/β, a type I IFN, is induced by interferon regulatory factors (IRFs) 3 and 7 and stimulates the JAK-STAT signal pathway via type I IFN-α/β receptors composed of the IFNAR1 and IFNAR2 subunits [15, 16]. At that time, activated JAK1 and tyrosine kinase 2 bind to the intracellular domain of IFNAR1/2 and are responsible for phosphorylating and dimerizing STAT1/2, which serves as a transcription factor [15, 16]. Phosphorylated and dimerized STAT1/2 combines with IRF9 to form the ISGF3 complex, which activates the transcription of ISGs by binding to the IFN-stimulated responsive element after nuclear translocation [15, 16].
MX is known to suppress several viruses including the influenza virus and vesicular stomatitis virus (VSV) [17,18,19,20]. In cows, two isoforms of MX are present—MX1 and MX2; additionally, bovine MX1 has alternative splice variants, MX1-a and MX1B [9, 21]. Interestingly, MX1-a has antiviral activity against VSV, whereas MX1B does not inhibit VSV proliferation in the cytoplasm, and its own function is unknown [22, 23]. In fact, MX1-a localizes in the cytoplasm, but almost all of MX1B localizes in the nucleus. MX2 also has antiviral activity against VSV [24], and its intracellular localization is unknown [25]. As mentioned above, cows have three MX gene isoforms; however, the distribution of MX1-a and MX1B expression have not been analyzed. In addition, very little is known about MX expression in uterine and placental tissue after implantation.
In this study, to elucidate whether these MX genes are expressed after implantation in cows, we investigated to detect the expression dynamics of MX1-a, MX1B and MX2 mRNA in uterine and placental tissues from early to mid pregnancy. Furthermore, we examined relative expressions of IFN-α mRNA and the expression of type I IFN receptor subunits (IFNAR1 and IFNAR2) and interferon regulatory factors (IRF3 and IRF9) in relation to the type I IFN signaling pathway.
Materials and Methods
Sample collection from pregnant uterine and fetal tissues
This study was conducted in accordance with the Hokkaido University guidelines for the care and use of animals. Pregnant uteri were collected from cows slaughtered between days 14 and 18 of pregnancy after embryo transfer on day 7 of the estrus cycle. Uteri at mid-pregnancy stages were collected from abattoirs. The collected pregnant uteri were divided into five groups: days 14–18 (n = 3), 25–40 (n = 3), 50–70 (n = 3), 80–100 (n = 4) and 130–150 (n = 4), confirming the existence of a conceptus on days 14–18 and assessing day of pregnancy in the other groups according to crown-rump length of fetuses from the top of the head to the bottom of the buttocks. The intercaruncular endometrium (IC) and caruncular endometrium (C) tissues from pregnant uterine horns, and fetal placenta (P) tissues were collected for RNA extraction.
RNA extraction and reverse transcription polymerase chain reaction (RT-PCR)
Total RNA was extracted from the 3 tissue groups (IC, C and P) using ISOGEN II (Nippon Gene, Toyama, Japan), and cDNA was synthesized from total RNA by reverse transcription using the ReverTra Ace® qPCR RT Master Mix with gDNA Remover (Toyobo Life Science, Osaka, Japan) according to the manufacturer’s protocol. PCR was performed with an Astec Program Temp Control System (PC-815 or 816, Astec, Fukuoka, Japan). Each cDNA sample concentration was measured by spectrophotometry (NanoDrop ND-2000, Thermo Scientific, Wilmington, DE, USA) and was adjusted to 100 ng/μl. All cDNA samples were stored in a freezer at –30 C.
RT-PCR and quantitative RT-PCR (qRT-PCR)
Specific primers for MX1-a, MX1B, MX2, IFN-α, IFNAR1, IFNAR2, IRF3, IRF9 and H2AFZ mRNA expression were designed using Primer-BLAST. The primer details are shown in Table 1. Expression of IFNAR1, IFNAR2, IRF3, IRF9 and H2AFZ was detected by RT-PCR analysis using an Astec Program Temp Control System (PC-815 or 816, Astec) and GoTaq® Hot Start Green Master Mix (Promega, Madison, WI, USA). The thermal cycling conditions were 1 cycle at 95 C for 5 min (initial denaturation); 35 cycles at 95 C for 30 sec (denaturation), 55 C for 1 min (primer annealing) and 72 C for 1 min (extension); and then 1 cycle at 72 C for 5 min (final extension). The PCR products were detected by electrophoresis using a 2.0% agarose gel with Midori Green (Nippon Genetics, Tokyo, Japan).
Table 1. Information about the primer sequences used for RT-PCR or qRT-PCR.
| Gene | Sequence (5’–3’) | Accession No. | Product length (bp) |
| MX1-a | GCCAACTAGTCAGCACTACATTGTC | NM_173940.2 | 139 |
| GCTCTTGGACTCCATATCTTCAC | |||
| MX1B | GTGATATCTCCAACAGTGAAGC | AB_060169.1 | 94 |
| AACTGATTCGAGAAGCCAAG | |||
| MX2 | CAGAGACGCCTCAGTCGAAG | NM_173941.2 | 113 |
| GAGACGTTTGCTGGTTTCCATG | |||
| IFN-α | CTAGAGAGCAGGTTCACAGAGTC | NM_001017411.1 | 106 |
| GCTGAGCAGCAACAGGGATAG | |||
| IFNAR1 | GCGAAGAGTTTCCGCAACAG | NM_174552.2 | 275 |
| TCCAAGGCAGGTCCAATGAC | |||
| IFNAR2 | TCGTATGTTGCGCCTGTTCT | NM_174553.2 | 231 |
| GTCCGTCGTGTTTACCCACA | |||
| IRF3 | GCTCAACTGACGGGAAGTGG | NM_001029845.3 | 116 |
| TGGTCTGGCCTAAGTGTTGG | |||
| IRF9 | CAGTTCCCAGGAGTGTGCTG | NM_001024506.1 | 125 |
| TATATCGCCCAGGCCTTGAA | |||
| H2AFZ | AGAGCCGGTTTGCAGTTCCCG | NM_174809.2 | 116 |
| TACTCCAGGATGGCTGCGCTGT |
The expression levels of MX1-a, MX1B, MX2 and IFN-α were invesitigated by qRT-PCR using a LightCycler® 480 System II (Roche Diagnostics, Basel, Switzerland) and THUNDERBIRDTM SYBR® qPCR Mix (Toyobo Life Science). The thermal cycling conditions were 1 cycle at 95 C for 30 sec (denaturation), followed by 50 cycles at 95 C for 10 sec (denaturation), 55 C for 15 sec (primer annealing) and 72 C for 30 sec (extension). The relative expression levels of MX1-a, MX1B, MX2 and IFN-α were calculated by the ΔΔCt method using the expression of H2AFZ as the reference gene.
Statistical analysis
All data are shown as the mean ± standard error of the mean (SEM). The statistical significance of differences was assessed by one-way analysis of variance (ANOVA) followed by the Fisher’s protected least-significant difference (PLSD) procedure as the multiple comparison test. P values of < 0.01 or < 0.05 were considered statistically significant. P values of < 0.1 was regarded as indicating a tendency.
Results
Expression of MX1-a, MX1B and MX2 mRNA in uterine tissues in the pre- and postimplantation stages
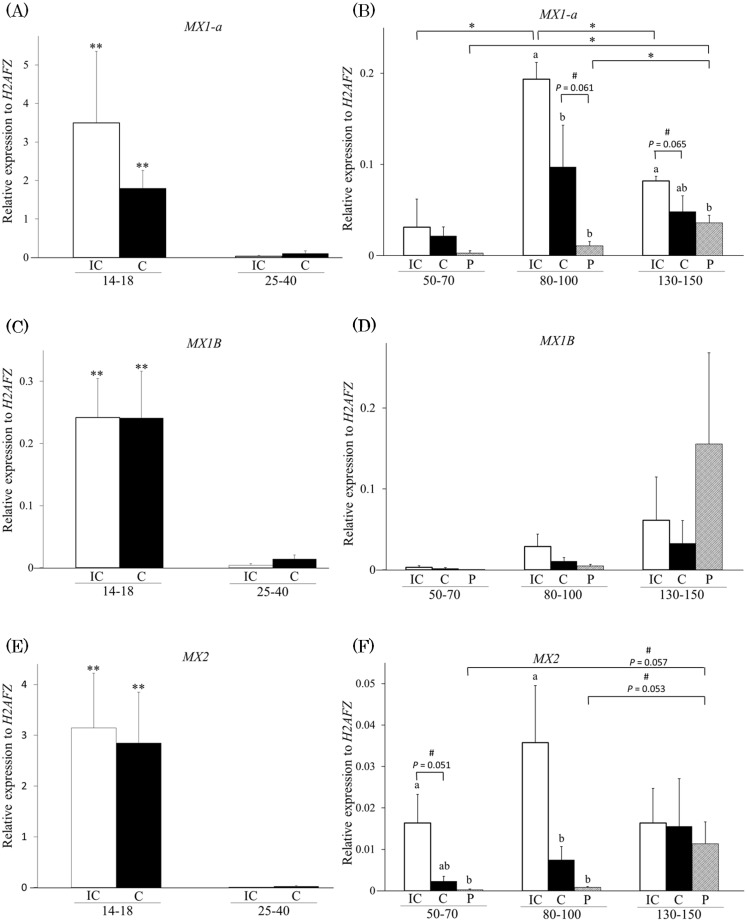
The relative expressions of MX1-a, MX1B and MX2 mRNA in pregnant uterine tissues (IC and C) were higher at days 14–18 than days 25–40 (Fig. 1A, C and E; P < 0.01) and the highest on days 14–18 compared with the mid-pregnancy stages, including days 50–70, 80–100 and 130–150 (Fig. 1 A–F; P < 0.01). Although the expression levels of all the MX mRNAs markedly decreased immediately after the pregnancy stage of days 25–40 in the time of implantation, these MX genes were also expressed in the endometrium after implantation.
Fig. 1.
Expression of MX1-a, MX1B, and MX2 mRNA in the endometrium and fetal placenta in the pre- and postimplantation stages. The vertical line shows the relative expression levels of mRNA using qRT-PCR, standardized with the reference gene H2AFZ, whereas the horizontal axis indicates the stages of pregnancy and types of tissue samples for the genes (A and B) MX1-a, (C and D) MX1B, and (E and F) MX2. IC, intercaruncular endometrium; C, caruncular endometrium; P, fetal placenta. The numbers 14–18, 25–40, 50–70, 80–100, and 130–150 indicate the number of pregnancy days for each subgroup. All data are shown as the mean ± standard error of the mean (SEM). Asterisks indicate significant differences (** P < 0.01; * P < 0.05) among pregnancy stages, and letters indicate significant differences (P < 0.05) among three tissues as determined by ANOVA, followed by the Fisher’s PLSD procedure as the multiple comparison test. Additionally, a number sign (#) indicates a tendency for significant differences (P < 0.1).
Expression of MX1-a, MX1B and MX2 mRNA in uterine and fetal placental tissues at mid pregnancy
Comparison of the expression levels in IC, C and P tissues revealed that MX1-a expression was significantly higher in IC tissue than in C or P tissues at days 80–100 and higher than in P tissues on days 130–150 (Fig. 1B; P < 0.05). The C and P tissues showed different tendencies with respect to expression at days 80–100, and the IC and C tissues showed different tendencies at days 130–150 (P < 0.1). MX2 expression in the IC tissues was higher than in the P tissue at days 50–70 and higher than in either C or P tissues at days 80–100 (Fig. 1F; P < 0.05). The expression of MX2 in the IC tissues also tended to be higher at days 50–70 in the C tissue (P < 0.1). No significant difference was observed in MX1B expression (Fig. 1D).
Comparison of the levels at days 50–70, 80–100 and 130–150 revealed that MX1-a expression in the IC tissue increased significantly at days 80–100 followed by a significant decrease (Fig. 1B; P < 0.05). The expression pattern of MX1-a and MX2 in the P tissue was similar; MX1-a expression in the P tissue at days 130–150 was significantly higher than at days 50–70 and 80–100 (Fig. 1B; P < 0.05), and MX2 expression in the P tissue at days 130–150 tended to be higher than at days 50–70 and 80–100 (Fig. 1F; P < 0.1).
Expressions of the mRNA of IFN-α and IFN pathway-related genes (IFNAR1, IFNAR2, IRF3 and IRF9) in uterine and fetal placental tissues during pregnancy
Comparison of the expression levels of IFN-α mRNA among days 14–18, 25–40, 50–70, 80–100 and 130–150 revealed that IFN-α showed a scattered gene expression pattern; nevertheless, its expression in the IC tissue was significantly higher (P < 0.05) at days 50–70 than at days 14–18, 25–40 and 130–150 (Fig. 2A). The mRNA expression level of IFN-α in the C was not significantly different (Fig. 2B). In the P tissue, IFN-α was only minimally expressed during mid pregnancy (Fig. 2C).
Fig. 2.
Expression of IFN-α mRNA in the endometrium and fetal placenta in the pre- and postimplantation stages. The vertical line shows the relative expression levels of mRNA using qRT-PCR, standardized with the reference gene H2AFZ, whereas the horizontal axis indicates the stages of pregnancy for (A) IFN-α in IC tissues, (B) IFN-α in C tissues and (C) IFN-α in P tissues, respectively. IC, intercaruncular endometrium; C, caruncular endometrium; P, fetal placenta. The numbers 14–18, 25–40, 50–70, 80–100 and 130–150 indicate the number of days of pregnancy for each subgroup. All data are shown as the mean ± standard error of the mean (SEM). Letters (P < 0.05) indicate significant differences determined by ANOVA, followed by the Fisher’s PLSD procedure as the multiple comparison test.
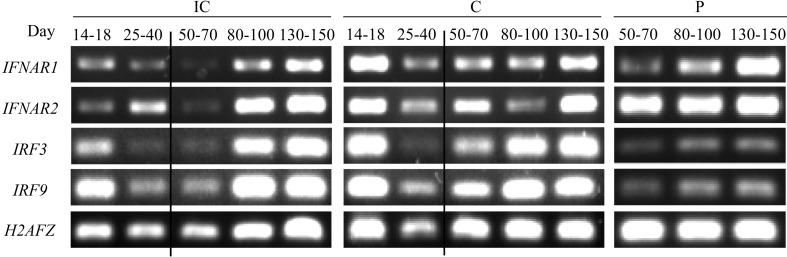
The expression of type I IFN receptors (IFNAR1 and IFNAR2) and type I IFN regulatory factors (IRF3 and IRF9) was detected in all pregnant uterine and fetal placental tissues by RT-PCR analysis (Fig. 3). Thus, not only IFN-a but also IFN signaling pathway-related genes were expressed in IC, C and P tissues during pregnancy.
Fig. 3.
Expression of IFNAR1, IFNAR2, IRF3 and IRF9 in the endometrium and fetal placenta in the pre- and postimplantation stages. The figure shows the expression of type I IFN receptor (IFNAR1 and IFNAR2) and type I IFN regulatory factor (IRF3 and IRF9) mRNA in pregnant uterine and placental tissues by RT-PCR. IC, intercaruncular endometrium; C, caruncular endometrium; P, fetal placenta. The numbers 14–18, 25–40, 50–70, 80–100 and 130–150 indicate the number of days of pregnancy for each subgroup. H2AFZ was used as the internal control gene.
Discussion
In this study, to determine whether bovine MX1-a, MX1B and MX2 genes were expressed in uterine and fetal placental tissues after implantation, we evaluated the expression of each MX mRNA in three different tissues (IC, C and P) during five pregnancy stages (days 14–18, 25–40, 50–70, 80–100 and 130–150) by qRT-PCR. MX1-a, MX1B and MX2 were expressed not only in the endometrium (IC and C) but also in the P at each pregnancy stage. The relative expression levels of each MX mRNA in both the IC and C were higher at days 14–18 than at the other stages. The expression of MX genes is induced by stimulation with IFN-τ, which is secreted from the trophoblast of the conceptus cells during pregnancy days 14 to 21 in ruminants [1]. MX genes induced by IFN-τ at preimplantation may play a role in pregnancy recognition or uterine reception. It has also been suggested that MX1 is involved in the immune response and in cell adhesion at implantation [10]. Therefore, MX genes may play a role in cell adhesion mechanisms between the mother and fetus, such as conceptus attachment to the endometrium.
Although MX expressions decreased from days 14–18 to days 25–40, these expressions continued to change during mid pregnancy. Since IFN-τ secretion by the conceptus decreases after establishment of implantation, it was thought that MX1-a, MX1B and MX2 mRNA after implantation might be induced by another type I IFN such as IFN-α/β. To determine whether MX expression correlated with the type I IFN signaling pathway, we focused on the mRNA of IFN-α, type I IFN receptors (IFNAR1 and IFNAR2) and type I IFN regulatory factors (IRF3 and IRF9). IFN-α mRNA was expressed in all pregnancy tissues. In particular, IFN-α expression in IC tissue was significantly higher at days 50–70. This result correlated with the upregulation of MX1-a and MX2 expression at days 80–100. IFN-α is transcribed by IRF3 and affects the JAK-STAT signaling pathway via IFNAR1 and IFNAR2 receptors, which is followed by expression of ISGs induced by IRF9 [15, 16]. Detection of IFNAR1, IFNAR2, IRF3 and IRF9 mRNA indicates that this cascade stimulates MX1-a, MX1B and MX2 mRNA even during pregnancy for the regulation of immune tolerance.
MX1-a, MX1B and MX2 mRNA were expressed in the IC, C, and P on days 50–70, 80–100, and 130–150 during the placental formation phase. When comparing tissues (IC, C and P), the relative expression levels of MX1-a (days 80–100 and 130–150) and MX2 (days 50–70 and 80–100) were significantly higher in the IC tissue than the P tissues. Thus, there was a tendency for the expression of the above genes to decrease in the P of fetal tissue than the IC of maternal tissue. Immune tolerance is required for successful pregnancy in any viviparous animal. In cows, decrease of expression of major histocompatibility proteins by the trophoblast, recruitment of macrophages to the uterus and modulation of immune-related genes contribute to the uterine immunosuppressive environment [26]. Immune tolerance between the mother and fetus is unbalanced when the uterus is infected with pathogens [27,28,29]. During pregnancy as well as non-pregnancy conditions, the TLRs—a major family of PRRs—are expressed in the uterus. The levels of TLR2, 3, 4, 6 and 9 are higher in the interplacentomal endometrium than in the placentome [26]. The present results suggest that pregnant cows may weakly modulate the immune response in the caruncular and placental tissues, where maternal tissue is in contact with fetal tissue; in contrast, a stronger immune response in the intercaruncular tissue that is not in contact with fetal tissue would protect the mother and fetus from pathogens.
Moreover, MX1-a and MX2 expression in the P tissue had a tendency to increase with the progress of pregnancy, although a significant difference was not recognized for MX1B expression, which has no antiviral activity against VSV. This indicates that immunity of the fetus may develop with the progression of pregnancy. Fetal immunity is formed from 80 to 120 days of pregnancy; hence, immune system maturation is not completed until about 120 days of gestation [30, 31]. Infection with bovine viral diarrhea virus prior to sufficient development of the fetal immune system causes abortion or calving of a persistent infected calf, which results in immunological tolerance [30, 31]. The expression of MX1-a in IC tissue increased at days 80–100, followed by a decrease at days 130–150. We suggest that the maternal innate immune response is active in the presence of an undeveloped fetal immune system until fetal immunity develops, at which time the maternal immune response then weakens.
Recently, it was shown that ovine MX1 protein was located within exosomes secreted by uterine epithelial cells [32]. Exosomes are extracellular vesicles containing and transporting proteins, mRNA and miRNA and are necessary for intercellular communication. Exosomes are mainly produced by immune, epithelial and tumor cells [33]. In humans, it has been shown that the syncytiotrophoblast cells in the placenta constitutively secrete exosomes during pregnancy [34, 35]. These exosomes, derived from the human placenta, are secreted into the maternal bloodstream and enable cross talk between the mother and child—namely, transport of substances such as proteins and RNA molecules [35]. In sheep, the exosomes secreted by uterine epithelial cells incorporate the MX1 protein [32]. This might also be applicable to cows in ruminants; bovine MX1 protein transported inside exosomes may play a role in fetal-maternal communication as well as in humans. The MX protein belongs to the dynamin superfamily of GTPases, and therefore, it is suggested that MX may play a role in intracellular transport and endocytosis in addition to having antiviral activity [36,37,38,39]. Ovine MX1 protein in uterine glandular epithelial cells interacts with tubulin β; this suggests that MX1 could play a role in transporting proteins or vesicles via secretion and mitosis [40]. MX may function in exosome secretion by uterine epithelial cells or serve as transporter.
Our findings reveal that three isoforms of bovine MX genes as well as the IFN signaling pathway-related genes, IFN-α, type I IFN receptors and type I IFN regulatory factors, are expressed in the IC, C and P tissues throughout pregnancy up to day 150. In the mid-pregnancy stages, the IC showed comparatively higher expressions of MX genes. Hence, in IC tissue that does not form a P, the mother regulates the immune response to defeat infections to protect the uterus and fetus. Consequently, it has been suggested that bovine MX genes are induced by IFN-α instead of IFN-τ after implantation, which indicates the need for the immune response to protect both the mother and embryo even during the placental development phase and maternal immune suppression.
Acknowledgments
This study was supported by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (KAKENHI, 15H04579). The authors thank the members of the Field Center for Northern Biosphere, Hokkaido University, for help with cow handling and sampling.
References
- 1.Ealy AD, Yang QE. Control of interferon-tau expression during early pregnancy in ruminants. Am J Reprod Immunol 2009; 61: 95–106. [DOI] [PubMed] [Google Scholar]
- 2.Demmers KJ, Derecka K, Flint A. Trophoblast interferon and pregnancy. Reproduction 2001; 121: 41–49. [DOI] [PubMed] [Google Scholar]
- 3.Spencer TE, Sandra O, Wolf E. Genes involved in conceptus-endometrial interactions in ruminants: insights from reductionism and thoughts on holistic approaches. Reproduction 2008; 135: 165–179. [DOI] [PubMed] [Google Scholar]
- 4.Dorniak P, Bazer FW, Spencer TE. Physiology and Endocrinology Symposium: biological role of interferon tau in endometrial function and conceptus elongation. J Anim Sci 2013; 91: 1627–1638. [DOI] [PubMed] [Google Scholar]
- 5.Bauersachs S, Wolf E. Immune aspects of embryo-maternal cross-talk in the bovine uterus. J Reprod Immunol 2013; 97: 20–26. [DOI] [PubMed] [Google Scholar]
- 6.Charleston B, Stewart HJ. An interferon-induced Mx protein: cDNA sequence and high-level expression in the endometrium of pregnant sheep. Gene 1993; 137: 327–331. [DOI] [PubMed] [Google Scholar]
- 7.Ott TL, Yin J, Wiley AA, Kim HT, Gerami-Naini B, Spencer TE, Bartol FF, Burghardt RC, Bazer FW. Effects of the estrous cycle and early pregnancy on uterine expression of Mx protein in sheep (Ovis aries). Biol Reprod 1998; 59: 784–794. [DOI] [PubMed] [Google Scholar]
- 8.Hicks BA, Etter SJ, Carnahan KG, Joyce MM, Assiri AA, Carling SJ, Kodali K, Johnson GA, Hansen TR, Mirando MA, Woods GL, Vanderwall DK, Ott TL. Expression of the uterine Mx protein in cyclic and pregnant cows, gilts, and mares. J Anim Sci 2003; 81: 1552–1561. [DOI] [PubMed] [Google Scholar]
- 9.Kojima T, Oshima K, Watanabe H, Komatsu M. The bovine Mx1 gene: characterization of the gene structure, alternative splicing, and promoter region. Biochem Genet 2003; 41: 375–390. [DOI] [PubMed] [Google Scholar]
- 10.Mansouri-Attia N, Aubert J, Reinaud P, Giraud-Delville C, Taghouti G, Galio L, Everts RE, Degrelle S, Richard C, Hue I, Yang X, Tian XC, Lewin HA, Renard J-P, Sandra O. Gene expression profiles of bovine caruncular and intercaruncular endometrium at implantation. Physiol Genomics 2009; 39: 14–27. [DOI] [PubMed] [Google Scholar]
- 11.Bauersachs S, Ulbrich SE, Reichenbach HD, Reichenbach M, Büttner M, Meyer HH, Spencer TE, Minten M, Sax G, Winter G, Wolf E. Comparison of the effects of early pregnancy with human interferon, alpha 2 (IFNA2), on gene expression in bovine endometrium. Biol Reprod 2012; 86: 46. [DOI] [PubMed] [Google Scholar]
- 12.Kim MS, Min KS, Imakawa K. Regulation of interferon-stimulated Gene (ISG)12, ISG15, and MX1 and MX2 by conceptus interferons (IFNTs) in bovine uterine epithelial cells. Asian-australas J Anim Sci 2013; 26: 795–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li J, Roberts RM. Interferon-tau and interferon-alpha interact with the same receptors in bovine endometrium. Use of a readily iodinatable form of recombinant interferon-tau for binding studies. J Biol Chem 1994; 269: 13544–13550. [PubMed] [Google Scholar]
- 14.Kawai T, Akira S. Toll-like receptor and RIG-I-like receptor signaling. Ann N Y Acad Sci 2008; 1143: 1–20. [DOI] [PubMed] [Google Scholar]
- 15.Zhou X, Michal JJ, Zhang L, Ding B, Lunney JK, Liu B, Jiang Z. Interferon induced IFIT family genes in host antiviral defense. Int J Biol Sci 2013; 9: 200–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Samuel CE. Antiviral actions of interferons. Clin Microbiol Rev 2001; 14: 778–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Staeheli P, Haller O, Boll W, Lindenmann J, Weissmann C. Mx protein: constitutive expression in 3T3 cells transformed with cloned Mx cDNA confers selective resistance to influenza virus. Cell 1986; 44: 147–158. [DOI] [PubMed] [Google Scholar]
- 18.Pavlovic J, Zürcher T, Haller O, Staeheli P. Resistance to influenza virus and vesicular stomatitis virus conferred by expression of human MxA protein. J Virol 1990; 64: 3370–3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Horisberger MA, Gunst MC. Interferon-induced proteins: identification of Mx proteins in various mammalian species. Virology 1991; 180: 185–190. [DOI] [PubMed] [Google Scholar]
- 20.Jin HK, Takada A, Kon Y, Haller O, Watanabe T. Identification of the murine Mx2 gene: interferon-induced expression of the Mx2 protein from the feral mouse gene confers resistance to vesicular stomatitis virus. J Virol 1999; 73: 4925–4930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ellinwood NM, McCue JM, Gordy PW, Bowen RA. Cloning and characterization of cDNAs for a bovine (Bos taurus) Mx protein. J Interferon Cytokine Res 1998; 18: 745–755. [DOI] [PubMed] [Google Scholar]
- 22.Nakatsu Y, Yamada K, Ueda J, Onogi A, Ables GP, Nishibori M, Hata H, Takada A, Sawai K, Tanabe Y, Morita M, Daikohara M, Watanabe T. Genetic polymorphisms and antiviral activity in the bovine MX1 gene. Anim Genet 2004; 35: 182–187. [DOI] [PubMed] [Google Scholar]
- 23.Yamada K, Nakatsu Y, Onogi A, Ueda J, Watanabe T. Specific intracellular localization and antiviral property of genetic and splicing variants in bovine Mx1. Viral Immunol 2009; 22: 389–395. [DOI] [PubMed] [Google Scholar]
- 24.Babiker HA, Nakatsu Y, Yamada K, Yoneda A, Takada A, Ueda J, Hata H, Watanabe T. Bovine and water buffalo Mx2 genes: polymorphism and antiviral activity. Immunogenetics 2007; 59: 59–67. [DOI] [PubMed] [Google Scholar]
- 25.Sasaki K, Kawahara M, Watanabe T. Characteristics of MX gene that shows resistance to vesicular stomatitis virus from avians to mammals. Advances in Genetics Research 2013; Chapter 6, 11: 117–130. [Google Scholar]
- 26.Oliveira LJ, Barreto RS, Perecin F, Mansouri-Attia N, Pereira FT, Meirelles FV. Modulation of maternal immune system during pregnancy in the cow. Reprod Domest Anim 2012; 47(Suppl 4): 384–393. [DOI] [PubMed] [Google Scholar]
- 27.Thaxton JE, Romero R, Sharma S. TLR9 activation coupled to IL-10 deficiency induces adverse pregnancy outcomes. J Immunol 2009; 183: 1144–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robertson SA, Care AS, Skinner RJ. Interleukin 10 regulates inflammatory cytokine synthesis to protect against lipopolysaccharide-induced abortion and fetal growth restriction in mice. Biol Reprod 2007; 76: 738–748. [DOI] [PubMed] [Google Scholar]
- 29.Krishnan L, Guilbert LJ, Wegmann TG, Belosevic M, Mosmann TR. T helper 1 response against Leishmania major in pregnant C57BL/6 mice increases implantation failure and fetal resorptions. Correlation with increased IFN-gamma and TNF and reduced IL-10 production by placental cells. J Immunol 1996; 156: 653–662. [PubMed] [Google Scholar]
- 30.Corapi WV, Donis RO, Dubovi EJ. Monoclonal antibody analyses of cytopathic and noncytopathic viruses from fatal bovine viral diarrhea virus infections. J Virol 1988; 62: 2823–2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McClurkin AW, Littledike ET, Cutlip RC, Frank GH, Coria MF, Bolin SR. Production of cattle immunotolerant to bovine viral diarrhea virus. Can J Comp Med 1984; 48: 156–161. [PMC free article] [PubMed] [Google Scholar]
- 32.Racicot K, Schmitt A, Ott T. The myxovirus-resistance protein, MX1, is a component of exosomes secreted by uterine epithelial cells. Am J Reprod Immunol 2012; 67: 498–505. [DOI] [PubMed] [Google Scholar]
- 33.Mincheva-Nilsson L, Baranov V. The role of placental exosomes in reproduction. Am J Reprod Immunol 2010; 63: 520–533. [DOI] [PubMed] [Google Scholar]
- 34.Hedlund M, Stenqvist AC, Nagaeva O, Kjellberg L, Wulff M, Baranov V, Mincheva-Nilsson L. Human placenta expresses and secretes NKG2D ligands via exosomes that down-modulate the cognate receptor expression: evidence for immunosuppressive function. J Immunol 2009; 183: 340–351. [DOI] [PubMed] [Google Scholar]
- 35.Luo SS, Ishibashi O, Ishikawa G, Ishikawa T, Katayama A, Mishima T, Takizawa T, Shigihara T, Goto T, Izumi A, Ohkuchi A, Matsubara S, Takeshita T, Takizawa T. Human villous trophoblasts express and secrete placenta-specific microRNAs into maternal circulation via exosomes. Biol Reprod 2009; 81: 717–729. [DOI] [PubMed] [Google Scholar]
- 36.Chen MS, Obar RA, Schroeder CC, Austin TW, Poodry CA, Wadsworth SC, Vallee RB. Multiple forms of dynamin are encoded by shibire, a Drosophila gene involved in endocytosis. Nature 1991; 351: 583–586. [DOI] [PubMed] [Google Scholar]
- 37.van der Bliek AM, Meyerowitz EM. Dynamin-like protein encoded by the Drosophila shibire gene associated with vesicular traffic. Nature 1991; 351: 411–414. [DOI] [PubMed] [Google Scholar]
- 38.Horisberger MA. Interferon-induced human protein MxA is a GTPase which binds transiently to cellular proteins. J Virol 1992; 66: 4705–4709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haller O, Stertz S, Kochs G. The Mx GTPase family of interferon-induced antiviral proteins. Microbes Infect 2007; 9: 1636–1643. [DOI] [PubMed] [Google Scholar]
- 40.Racicot K, Ott T. The myxovirus resistance protein, MX1, interacts with tubulin beta in uterine glandular epithelial cells. Am J Reprod Immunol 2011; 65: 44–53. [DOI] [PubMed] [Google Scholar]