Abstract
AML relapse remains the leading cause of transplant failure among Allo-SCT recipients. A single institution study was conducted on 348 patients with AML who received an Allo-SCT from an umbilical cord blood (UCB, 222) or HLA-matched related (RD, 126) donor between 2000–2011. Relapse after Allo-SCT occurred in 72 UCB and 32 RD transplant recipients. Three patients achieved CR after withdrawal of immune suppression with no further therapy. Fifty-two patients received intensive post-relapse therapy, defined as systemic chemotherapy (22 UCB, 7 RD), second Allo-SCT (9 UCB, 2 RD), or DLI ± systemic chemotherapy (0 UCB, 12 RD); of these, 25% achieved CR (21% UCB vs. 35% RD, p=0.16). Survival at 1 year after relapse was 22% for all patients (19% UCB vs. 28% RD, p=0.36). In multivariable analysis, post-relapse mortality was lower in patients receiving intensive therapy for relapse (HR=0.4; 95% CI 0.2–0.6, p<0.01) and higher in patients with peripheral blood blasts above the median (HR=3.8; 95% CI 2.2–6.6, p<0.01), active infection (HR=1.9; 95% CI 1.0–3.5, p=0.05), and non-infectious medical complications (HR=2.0; 95% CI 1.2–3.5, p=0.01). In conclusion, patients with AML relapsing after Allo-SCT who were in good enough clinical condition to receive intensive therapy had superior short-term survival.
Keywords: AML, Transplantation, Relapse, DLI, Second transplant, Survival
INTRODUCTION
Acute myeloid leukemia (AML) relapse remains the leading cause of transplant failure among allogeneic hematopoietic cell transplant (Allo-SCT) recipients. Patients experiencing leukemia relapse after Allo-SCT have limited treatment options and their prognosis remains poor even for individuals who can tolerate further therapies.1–5 Common therapeutic strategies for patients with post-Allo-SCT relapse include withdrawal of immunosuppression, conventional cytoreductive chemotherapies, adoptive immunotherapy using donor lymphocyte infusion (DLI), or a second Allo-SCT. These treatment options are influenced by graft source, post-transplant remission interval, leukemia burden, presence of infection, active graft vs. host disease (GVHD), and performance status. Notably, while DLI is commonly available for patients receiving allograft from related donors, it is not available for umbilical cord blood (UCB) transplant recipients.
The purpose of this study was to identify patient, disease, and treatment factors affecting clinical outcomes in AML patients who relapse after Allo-SCT, with the ultimate goal of identifying patients who could potentially benefit from therapeutic interventions. In addition, we did an exploratory analysis investigating whether the unavailability of DLI in UCB recipients adversely impacted outcomes as compared to related donor (RD) recipients.
PATIENTS AND METHODS
Patients
In this study patients with AML were included if they had received an RD or UCB Allo-SCT at the University of Minnesota from 2000 to 2011. Recipients of both myeloablative and reduced intensity conditioning (RIC) regimens were included. For patients receiving their initial remission induction chemotherapy at outside facilities, the diagnosis of AML was independently confirmed prior to transplantation by a hematopathologist at the University of Minnesota. 6, 7 Metaphase karyotyping was used for cytogenetic analysis, and the cytogenetic risk group was stratified according to the Medical Research Council (MRC) classification system. 8 The conditioning regimen, immunosuppressive regimens, and supportive care of this study population have been previously reported.9–11
Data Source and Study Definitions
Patient characteristics, transplant-related factors, and outcome data were prospectively collected and recorded in the Blood and Marrow Transplant database, which contains data on all patients receiving Allo-SCT at the University of Minnesota. Patient charts were used to retrospectively abstract additional information on patient characteristics at the time of relapse including immunosuppressive therapy status, withdrawal of immunosuppression, active GVHD, infection, other medical complications, and treatment type at relapse. Early and late relapse was defined as remission duration from transplant to AML relapse of <6 months or ≥6 months, respectively. Management of patients at relapse was categorized as intensive therapy or supportive care. Intensive therapy was defined as treatment with chemotherapy alone, DLI ± chemotherapy, or second transplant. Supportive care was defined as withdrawal of immunosuppression, hydroxyurea, transfusion support, and symptom management. AML relapse was defined as morphological evidence of leukemia recurrence.
Statistical Analysis
UCB and RD groups were compared using the chi-square test for categorical factors and the Wilcoxon signed rank test for continuous factors. One-year survival was calculated by Kaplan-Meier and compared by the log rank test. Univariable and multivariable Cox regression was used to estimate the mortality hazard ratio (HR) for selected clinical factors from relapse until three years after relapse. Univariable and multivariable logistic regression was used to estimate the odds ratio (OR) of not achieving complete remission (i.e., an OR >1 represents a worse outcome). For multivariable models, an iterative forward variable selection procedure was used with a p-value cutoff of 0.15 to select a subset of factors that may be predictive of an outcome. Donor type was included in multivariable models regardless of statistical significance. Only factors that were present at the time of relapse were considered for all regression models. For some factors, up to 20% of the patient values were unknown, and patients were excluded from any model that contained a factor for which the patient’s status was unknown. All statistical analysis was done with SAS software (Cary, NC, Version 9.2).
RESULTS
Patient Characteristics
Of the 348 patients with AML who received Allo-SCT, 104 relapsed after transplantation: 32 of 126 (25.4%) RD transplant recipients and 72 of 222 (32.4%) UCB recipients (Table 1). Relapse occurred twice as often among recipients of RIC than among recipients of myeloablative conditioning (40.5% vs. 21.5%). Median time from transplant to relapse was 152 days (interquartile range (IQR), 77–322 days) for all patients and was similar for both donor types. Median age of patients was 51 years (range, 1–71), and about one third of patients had unfavorable cytogenetics. Peripheral blood graft source was used for the majority of RD transplants (n=28; 88%). Although UCB and RD groups had similar demographic and disease-related characteristics, UCB recipients were more likely to undergo RIC transplantation (68% vs. 41%, p=0.01) and had more active infections at the time of relapse (25% vs. 6%, p=0.03) than RD transplant recipients. Notably, non-infectious medical complications (i.e., heart failure and arrhythmias, liver, lung and kidney injury) at relapse were also more frequent in UCB recipients (33% vs. 13%, p=0.04) than in RD transplant recipients. In contrast, the two donor types were similar in terms of the manifestation of leukemia relapse (i.e., systemic vs. extramedullary), chimerism, peripheral blood and bone marrow blast percentage at relapse, prevalence of GVHD, and the proportion of patients on immunosuppressive therapy.
Table 1.
Patient characteristics
| Patient characteristics | Total N=104 n (%) |
MRD N=32* n (%) |
UCB N=72 n (%) |
P-value |
|---|---|---|---|---|
|
| ||||
| Age | .67 | |||
| Median (IQR) | 51 (31–59) | 49 (35–56) | 52 (29–60) | |
|
| ||||
| Gender | .79 | |||
| Male | 54 (52) | 16 (50) | 38 (53) | |
|
| ||||
| Cytogenetic risk | ||||
| Unfavorable | 33 (32) | 12 (38) | 21 (29) | .40 |
|
| ||||
| Conditioning regimen | .01 | |||
| Myeloablative | 42 (40) | 19 (59) | 23 (32) | |
| Reduced intensity | 62 (60) | 13 (41) | 49 (68) | |
|
| ||||
| Days from SCT to relapse, | .98 | |||
| Median (IQR) | 152 (77–322) | 104 (87–339) | 168 (74–319) | |
|
| ||||
| Relapse site | .86 | |||
| Systemic | 88 (85) | 28 (87) | 60 (84) | |
| Extramedullar | 16 (15) | 4 (13) | 12 (17) | |
|
| ||||
| BM blast % | .82 | |||
| Median (IQR) | 12 (4–50) | 9 (3–63) | 15 (5–44) | |
|
| ||||
| PB blast % | .17 | |||
| Median (IQR) | 3 (0–15) | 5 (0–15) | 1 (0–20) | |
|
| ||||
| BM donor chimerism | .84 | |||
| Median (IQR) | 69 (32–94) | 78 (47–91) | 63 (32–95) | |
|
| ||||
| Infection at relapse | .03 | |||
| Yes | 20 (19) | 2 (6) | 18 (25) | |
| No | 71 (68) | 25 (78) | 46 (64) | |
| Unknown | 13 (13) | 5 (16) | 8 (11) | |
|
| ||||
| GVHD at relapse | .73 | |||
| Yes | 27 (26) | 7 (21) | 20 (28) | |
| No | 68 (65) | 20 (63) | 48 (67) | |
| Unknown | 9 (9) | 5 (16) | 4 (6) | |
|
| ||||
| Complications at relapse† | .04 | |||
| Yes | 28 (27) | 4 (13) | 24 (33) | |
| No | 62 (60) | 22 (69) | 40 (56) | |
| Unknown | 14 (13) | 6 (19) | 8 (11) | |
|
| ||||
| IST‡ at relapse | .91 | |||
| Yes | 62 (60) | 17 (53) | 45 (63) | |
| No | 28 (27) | 8 (25) | 20 (28) | |
| Unknown | 14 (13) | 7 (22) | 7 (9) | |
Graft source, peripheral blood N=28 (88%)
Heart failure, arrhythmias, liver, lung, and kidney injury
IST, immunosuppressive therapy
Management at Relapse
At relapse 62 patients were receiving immunosuppressive therapy, and information on further immunosuppression management after relapse was available for 59 subjects (Table 2). Nearly all patients regardless of donor type were tapered off of immunosuppressive therapy. Supportive care was used for one third of patients. UCB recipients were more likely to be managed supportively than RD transplant recipients (42% vs. 22%; p<0.01). Intensive therapy was used in about half of patients and consisted of systemic chemotherapy alone (n=29), DLI with or without cytoreductive chemotherapy (n=12), or second allogeneic transplant (n=11). Among patients receiving intensive therapy after post-Allo-SCT relapse, UCB recipients were more likely to receive systemic chemotherapy alone (29% vs. 19%, p<0.01) or a second allograft (13% vs. 6%, p<0.01). A second transplant was performed in 6 pediatric (age range 5–16 years) and 5 adult (age range 48–68) patients. All but one received a UCB graft for his or her initial transplant. All patients received their second allograft from a different donor. Five of 6 pediatric patients underwent a second Allo-SCT after receiving cytoreductive chemotherapy and achieving complete remission, and they all received a UCB graft. Among these 5 patients only one remained alive and leukemia free at last follow up of 3 years after second allograft for AML relapse. The rest of 4 patients died within 6 months of second SCT: 2 from recurrent leukemia, 1 from non-infectious pulmonary complication and the other 1 from bleeding and organ toxicity. The remaining one pediatric patient, who had low tumor burden (2% bone marrow blasts) at the time of leukemia relapse underwent second Allo-SCT without preceding intensive chemotherapy. However, her leukemia progressed in early post-transplant period resulting into fatal outcome within 2 months of transplantation. In contrast, only 1 of 5 adult patients underwent a second Allo-SCT while in complete remission after chemotherapy and local radiation for extramedullary myeloid sarcoma. This single adult patient received an RIC unrelated donor graft for relapsed AML 6 years after his initial RIC UCB allograft and remains in remission 4 years later. The remaining 4 adult patients received RIC haploidentical natural killer (NK) cell therapy followed by haploidentical transplant for their relapsed/persistent AML, as described previously by our group.12 Among these patients 2 died of leukemia recurrence within 3 months of their second allograft and 2 from infectious complications.
Table 2.
Management at Relapse
| Total N=104 n (%) |
RD N=32 n (%) |
UCB N=72 n (%) |
P-value | |
|---|---|---|---|---|
|
| ||||
| Withdrawal of IST* | .51 | |||
| Yes | 57 (75) | 16 (67) | 41 (79) | |
| No | 2 (3) | 1 (4) | 1 (2) | |
| Unknown | 17 (22) | 7 (29) | 10 (19) | |
|
| ||||
| Treatment at relapse | < .01 | |||
| Intensive | ||||
| Chemotherapy | 29 (28) | 7 (22) | 22 (31) | |
| DLI | 12 (12) | 12 (38) | 0 (0) | |
| 2nd AlloHCT | 11 (11) | 2 (6) | 9 (13) | |
| Supportive | 37 (34) | 7 (22) | 30 (42) | |
| Unknown | 15 (14) | 4 (13) | 11 (15) | |
Abbreviations: IST, immunosuppressive therapy
At relapse only 76 patients have been receiving IST
No information was available on the treatment of 15 patients managed elsewhere at the time of relapse. Most of these patients were also of unknown status for other risk factors at relapse.
Clinical Outcomes after Post-transplant Relapse
Complete remission after post-transplant relapse was achieved in only 25% of patients, and the 1-year survival rate for all patients was 22% (Table 3). In RD transplant and UCB recipients, complete remission (35% vs. 21%; p=0.16) and 1-year post-relapse survival rates (28% vs. 19%; p=0.32; Figure 1) were similar. The minimum follow-up time among survivors was one year. Notably, 3 (2 in UCB and 1 in RD groups) of 37 patients who were managed supportively achieved CR after withdrawal of immune suppression and no additional therapy. Relapse presentation was similar in these 3 patients who relapsed early in their post-transplant period (<100 day) and had minimal leukemia burden (<7% bone marrow and <1% peripheral blood circulating blasts) and high peripheral blood donor chimerism (>90%) at relapse. Among all patients managed supportively, only 4 remained alive 1 year after post-transplant relapse, of whom 3 were aforementioned patients achieving CR with withdrawal of immunosuppression alone. Only 1 of those patients is still alive and leukemia free 7 years after post-transplant relapse. This patient had transient flare of aGVHD after cessation of cyclosporine at relapse, however his GVHD was successfully managed with short-term course of steroid taper without any major further complications. Second patient had no GVHD prior to his day 100 AML relapse and he underwent successful withdrawal of immunosuppression with no immediate GVHD complication. However, a year later he developed extensive cGVHD and died 3 years after post-transplant relapse from complications of cGVHD and pulmonary fibrosis while still remaining in remission. The third patient was on steroid taper and cyclosporine for treatment of grade II GI aGVHD at the time of AML relapse. His cyclosporine was discontinued and GVHD treatment was continued with shorter course of steroid taper without any further GVHD flare. Unfortunately, a year later this patient was found to have extramedullary relapse of his AML which resulted into his death within next 2 months. There was one additional UCB transplant recipient among all patients in supportive care group who lived longer than a year after AML relapse. Although he was not able to achieve remission, he remained alive for 15 months after relapse with blood product transfusion and prophylactic antibiotic support only. Not unexpectedly, as compared to patients receiving supportive care, patients receiving intensive therapy had superior complete remission (44% vs. 8%, p<0.01) and 1-year survival (37% vs. 11%, p<0.01; Table 3). Among patients receiving intensive therapy, the CR rate was 28%, 58%, and 73% (p=0.03) in patients treated with chemotherapy alone, DLI ± systemic chemotherapy, or second transplant, respectively; there was no survival difference (35%, 50%, and 27%; p=0.51) among these three treatment approaches (Figure 2). In addition, CR was lower in the early relapse (<6 months, n=62) group than in the late relapse (≥6 months, n=42) group (20% vs. 41%; p=0.03); however, 1-year survival rates were similar in these groups (21% vs. 24%; p=0.12; Figure 3).
Table 3.
Clinical outcomes after post-transplant relapse
| Remission | One year Survival | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| CR rate (%) | 95% CI | p-value | 1-year Survival rate (%) | 95% CI | p-value | |
|
| ||||||
| Total | 25 | (17–34) | 22 | (15–31) | ||
|
| ||||||
| Donor type | .16 | .36 | ||||
| RD | 35 | (19–53) | 28 | (14–44) | ||
| UCB | 21 | (12–32) | 19 | (11–29) | ||
|
| ||||||
| Treatment type | < .01 | < .01 | ||||
| Intensive | 44 | (30–59) | 37 | (24–50) | ||
| Chemotherapy | 28 | (13–47) | 35 | (18–52) | ||
| DLI | 58 | (28–85) | 50 | (21–74) | ||
| 2nd AlloHCT | 73 | (39–94) | 27 | (7–54) | ||
| Supportive | 8 | (2–22) | 11 | (3–23) | ||
|
| ||||||
| Time to Relapse | .03 | .12 | ||||
| <6 months | 20 | (10–34) | 21 | (12–32) | ||
| ≥6 months | 41 | (26–58) | 24 | (13–38) | ||
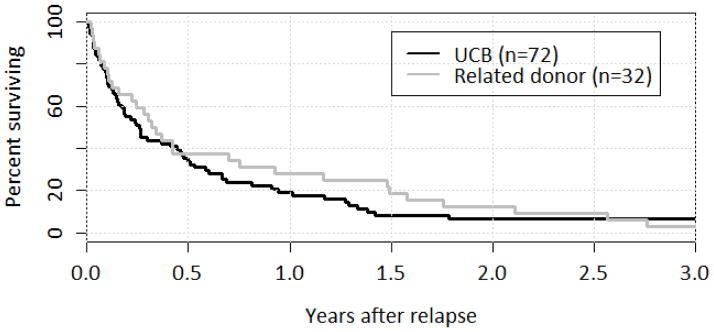
Figure 1.
Survival after Relapse by Donor Type
UCB and related donor survival curves over the first year following relapse were similar (p=0.36); at one year, OS was 28% for UCB and 19% for related donor transplants. By three years after relapse, less than 10% in either group were alive.
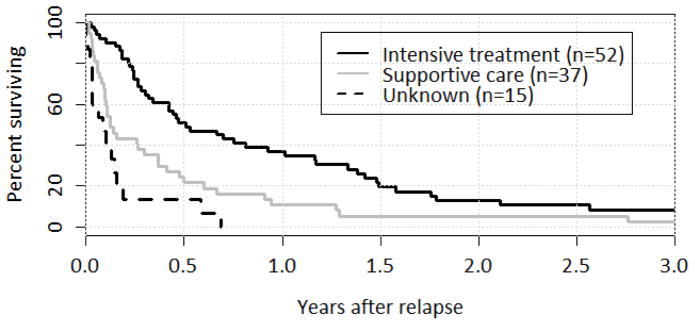
Figure 2.
Survival after Relapse by Treatment Type
Survival over the first year following relapse was higher in patients receiving intensive treatment (p < 0.01). At one year, OS was 37% following intensive therapy and 11% following supportive care. Of note, patients who were managed at other centers at the time of relapse and whose treatment could not be determined did worse than the known supportive care group.
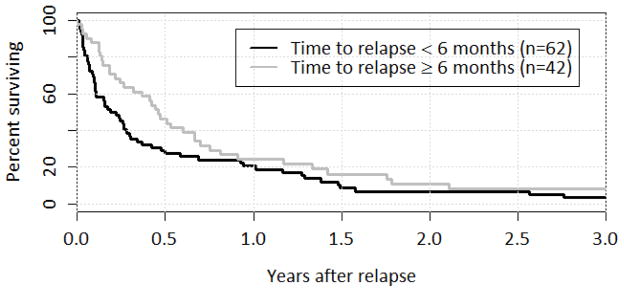
Figure 3.
Survival after Relapse by Time to Relapse
Survival over the first year was marginally higher for patients with a longer time to relapse (p=0.12), although by one year there was only a small difference between the groups: 24% OS for patients who relapsed six months or more after first transplant and 21% OS for those who relapsed within six months.
Remission after Post-transplant Relapse
In univariate analysis of remission after post-transplant relapse, patients who were older and those receiving immunosuppressive therapy at the time of relapse were less likely to achieve subsequent remission (Table 4). As expected, a higher CR rate was observed in patients treated with intensive therapy than in those managed supportively. Factors that did not predict subsequent remission after post-transplant relapse included donor type, unfavorable cytogenetics, intensity of conditioning regimen, time to relapse, percentage of peripheral blood and bone marrow blasts at relapse, donor chimerism, site of leukemia relapse, presence of active GVHD, and infectious and other medical complications. In multivariate analysis, receipt of intensive therapy was the only factor that remained prognostic for post-transplant remission.
Table 4.
Remission after post-transplant relapse
| Variable | Univariable | Multivariable (n=87) | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| OR* | 95% CI | p-value | HR | 95% CI | p-value | |
|
| ||||||
| Related donor | 0.5 | 0.2–1.3 | .16 | 1.1 | 0.3–4.5 | .94 |
|
| ||||||
|
Age Per 10 year increase |
1.3 | 1.1–1.7 | .01 | -- | -- | -- |
|
| ||||||
| Female | 1.2 | 0.5–3.0 | .69 | -- | -- | -- |
|
| ||||||
|
Cytogenetic risk Unfavorable |
1.0 | 0.4–2.8 | .93 | -- | -- | -- |
|
| ||||||
| Myeloablative conditioning | 0.5 | 0.2–1.2 | .13 | -- | -- | -- |
|
| ||||||
|
Time to relapse Per 100 day increase |
0.9 | 0.8–1.0 | .16 | -- | -- | -- |
|
| ||||||
| Systemic relapse | 1.5 | 0.4–4.9 | .53 | -- | -- | -- |
|
| ||||||
| BM blast % (Above median) † | 1.3 | 0.5–3.4 | .59 | -- | -- | -- |
|
| ||||||
| PB blast % (Above median) ‡ | 1.4 | 0.5–3.8 | .46 | -- | -- | -- |
|
| ||||||
|
BM donor chimerism Per 10% increase |
0.9 | 0.7–1.0 | .14 | -- | -- | -- |
|
| ||||||
| Infection at relapse | 1.6 | 0.5–5.6 | .43 | -- | -- | -- |
|
| ||||||
| GVHD at relapse | 2.4 | 0.7–8.1 | .14 | -- | -- | -- |
|
| ||||||
| Complications at relapse | 2.0 | 0.6–6.1 | .23 | -- | -- | -- |
|
| ||||||
| IST at relapse | 3.0 | 1.1–8.1 | .03 | -- | -- | -- |
|
| ||||||
| Intensive treatment (vs. supportive) | 0.1 | 0.0–0.4 | < .01 | 0.1 | 0.0–0.4 | < .01 |
|
| ||||||
| Treatment (vs. chemotherapy) | ||||||
| DLI | 0.3 | 0.1–1.2 | .10 | 0.3 | 0.0–1.7 | .17 |
| 2nd AlloHCT | 0.2 | 0.0–0.8 | .02 | 0.2 | 0.0–0.8 | .02 |
| Supportive | 4.8 | 1.1–20.2 | .03 | 4.8 | 1.1–20.3 | .03 |
An odds ratio (OR) greater than 1.0 is indicative of a decreased likelihood of achieving remission.
Median=12%
Median=3%
Mortality after Post-transplant Relapse
In univariate analysis of mortality after post-transplant relapse, risk of mortality was reduced in patients with late post-transplant relapse and those receiving intensive therapy (Table 5). There was no independent effect of donor type or intensity of conditioning regimen. Mortality was higher among individuals who had higher peripheral blood and bone marrow blasts, or active GVHD at relapse. In multivariate analysis, mortality was 3.8-fold higher in patients with >3% circulating peripheral blasts, 1.9-fold higher in patients with active infection, and 2-fold higher in patients with other medical complications at relapse. Patients receiving any type of intensive therapy as compared to supportive care experienced on average a 60% reduced mortality rate. Type of intensive therapy was not predictive of survival. A post-hoc regrouping showed no difference in overall survival for DLI and second SCT combined relative to chemotherapy (HR 0.9; 95% CI: 0.5–1.9; p=0.88).
Table 5.
Mortality following post-transplant relapse
| Variable | Univariable | Multivariable (n=79) | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| HR | 95% CI | p-value | HR | 95% CI | p-value | |
|
| ||||||
| Related donor | 0.8 | 0.5–1.3 | .38 | 0.9 | 0.5–1.6 | .71 |
|
| ||||||
|
Age Per 10 year increase |
1.0 | 0.9–1.1 | .57 | -- | -- | -- |
|
| ||||||
| Female | 1.2 | 0.8–1.8 | .41 | -- | -- | -- |
|
| ||||||
|
Cytogenetic risk Unfavorable |
1.1 | 0.7–1.7 | .56 | -- | -- | -- |
|
| ||||||
| Myeloablative conditioning | 1.0 | 0.6–1.4 | .82 | -- | -- | -- |
|
| ||||||
|
Time to relapse Per 100 day increase |
0.9 | 0.9–1.0 | .05 | -- | -- | -- |
|
| ||||||
| Systemic relapse | 1.2 | 0.7–2.1 | .57 | -- | -- | -- |
|
| ||||||
| BM blast % (Above median) † | 1.9 | 1.2–3.0 | < .01 | -- | -- | -- |
|
| ||||||
| PB blast % (Above median) ‡ | 2.6 | 1.6–4.1 | < .01 | 3.8 | 2.2–6.6 | < .01 |
|
| ||||||
|
BM donor chimerism Per 10% increase |
1.0 | 0.9–1.1 | .54 | -- | -- | -- |
|
| ||||||
| Infection at relapse | 1.6 | 0.9–2.7 | .09 | 1.9 | 1.0–3.5 | .05 |
|
| ||||||
| GVHD at relapse | 1.6 | 1.0–2.6 | .04 | |||
|
| ||||||
| Complications at relapse | 1.4 | 0.9–2.2 | .16 | 2.0 | 1.2–3.5 | .01 |
|
| ||||||
| IST at relapse | 1.4 | 0.9–2.3 | .14 | -- | -- | -- |
|
| ||||||
| Intensive treatment (vs. supportive) | 0.5 | 0.3–0.7 | < .01 | 0.4 | 0.2–0.6 | < .01 |
|
| ||||||
| Treatment (vs. chemotherapy) | ||||||
| DLI | 0.8 | 0.4–1.6 | .48 | 0.9 | 0.3–2.2 | .75 |
| 2nd AlloHCT | 0.8 | 0.4–1.7 | .53 | 1.0 | 0.4–2.5 | .94 |
| Supportive | 1.8 | 1.1–3.1 | .02 | 2.7 | 1.4–5.0 | < .01 |
Median=12%
Median=3%
Causes of death (n= 96) included leukemia relapse (n=88; 85%), infection (n=3; 3%), GVHD (n=2; 2%), bleeding/organ toxicity (n= 2; 2%), and non-infectious pulmonary complication (n= 1; 1%). Among deaths in chemotherapy treated group (n=24), 23 were from leukemia relapse and 1 from bacterial infection. All but one patient in DLI group (n=12) died from leukemia relapse, and cGVHD was the cause of death for that single patient. Among fatal cases in second transplant group (n=9) 5 were from leukemia relapse, 2 from infection, 1 from non-infectious pulmonary complication and the other 1 from bleeding/organ toxicity. Thirty-four patients in supportive care group died from relapsed leukemia; one patient died from bleeding and another one from cGVHD. Leukemia relapse was the cause of death for all patients (n=15) with unknown treatment who were managed elsewhere at the time of relapse.
DISCUSSION
Despite the curative therapeutic potential of Allo-SCT, survival of AML patients relapsing after transplantation is dismal. Treatment strategies for patients with post-Allo-SCT relapse is guided by patient characteristics and clinical factors. In general, patients who are older and those with comorbid conditions, active infections, and GVHD at relapse are more likely to be managed with supportive care. In contrast, young and otherwise healthy individuals generally receive further intensive therapy. In cases of morphological AML relapse, withdrawing immunosuppression to induce a graft versus leukemia (GVL) effect is routinely attempted; however, our data demonstrated that withdrawal of immunosuppression alone was unlikely to provide clinical benefit in such cases, a conclusion reached in previous reports as well. 13, 14 In our study, patients treated with withdrawal of immunosuppression alone rarely achieved CR and had the worst survival. In part, this group may also have been judged unfit for other therapies at the time of relapse. Our study found that about half of patients were managed with intensive therapy, and these patients had superior rates of remission and survival as compared to patients receiving supportive care. In patients receiving cytoreductive chemotherapy, CR was attained in about one third of cases. This observation is consistent with previous studies demonstrating a 30–40% remission rate in patients receiving salvage chemotherapy for relapsed leukemia after Allo-SCT.13, 15 Interestingly, our data also demonstrated that patients who received chemotherapy alone had similar survival to those undergoing DLI or a second transplantation, though the number of patients in each treatment group was too limited to be definitive.
DLI is another intensive therapeutic option available after adult donor transplantation, but not for recipients of UCB grafts. In our study, half of RD DLI recipients were alive at 1 year. This is consistent with EBMT data on the efficacy of DLI in AML. 4 Two-year survival for all patients treated with DLI in the EBMT study was 21% as compared to 56% for the subgroup achieving CR with chemotherapy prior to DLI. 4 Other investigators also reported comparable results with DLI use in AML patients relapsing after transplantation. 16–20 Because DLI is not an option for UCB recipients, chemotherapy with or without second Allo-SCT is used for relapse whenever possible. Interestingly, lack of the DLI option for UCB recipients in our study did not negatively influence their OS, which could be partially explained by more frequent use of a second allograft for relapsed leukemia in UCB recipients as compared to RD recipients.
A recent study based on EBMT data showed a 2-year survival of 60% in patients receiving a second transplant in CR. This long-term survival rate was achieved in the subgroup of patients who achieved CR with cytoreductive chemotherapy followed by cell-based therapy. 1 Unlike some reports of a second transplant providing the best outcome for patients with post-transplant relapse1, 13, 15, the data from our study demonstrated a modest survival benefit after second allograft, even though the CR rate was over 70% in individuals undergoing a second transplantation. Relapse, but not the treatment related complications, was the main cause of death even for those receiving intensive therapy. Overall, despite achievement of moderately successful remission rates with available intensive therapy options, our results are comparable with most existing literature showing non-sustained responses or remissions in the large majority of cases and poor overall survival for AML that relapsed after Allo-SCT. 1, 13, 14 Additionally, we identified no subsequent remission or survival differences based on time to relapse or intensity of the initial allograft conditioning regimen. Some 3, 21 reports suggest that the outcome of leukemia relapse in RIC transplants is different from myeloablative Allo-SCT 1, 15, 22, 23. As suggested by Schmid and colleagues, leukemia relapse after RIC does not necessarily indicate failure of the GVL effect, and cytoreductive chemotherapy can potentially help to facilitate the GVL effect by reducing leukemia burden. 1 Given that more UCB patients in our study underwent RIC transplantation, we speculate that the long lasting responses to chemotherapy among some of our study patients may have been partially due to enhancement of the GVL effect. Alternative explanation is that in some cases of AML relapse after transplantation leukemic cells escape donor lymphocyte antileukemic activity by acquiring additional mutations. 24
In summary, despite favorable clinical responses and remission rates, overall survival for patients whose AML relapses after Allo-SCT was poor, with a 1-year survival of only 22% for all patients. Although survival with currently available therapeutic options is disappointing, we recommend offering intensive therapy to patients who are considered clinically fit because of the significant survival benefit achieved with intensive treatment options as compared to immunosuppression withdrawal or supportive care alone. Whenever possible, participation in clinical trials that explore therapeutic and preventive strategies should be encouraged for these patients given the overall dismal clinical outcomes after relapse.
Acknowledgments
We would like to acknowledge Michael Franklin, MS, for assistance in editing this manuscript.
Footnotes
Authors have no relevant conflict of interest to disclose
References
- 1.Schmid C, Labopin M, Nagler A, Niederwieser D, Castagna L, Tabrizi R, et al. Treatment, risk factors, and outcome of adults with relapsed AML after reduced intensity conditioning for allogeneic stem cell transplantation. Blood. 2012;119(6):1599–606. doi: 10.1182/blood-2011-08-375840. [DOI] [PubMed] [Google Scholar]
- 2.Devillier R, Crocchiolo R, Etienne A, Prebet T, Charbonnier A, Furst S, et al. Outcome of relapse after allogeneic stem cell transplant in patients with acute myeloid leukemia. Leukemia & lymphoma. 2013;54(6):1228–34. doi: 10.3109/10428194.2012.741230. [DOI] [PubMed] [Google Scholar]
- 3.Savani BN, Mielke S, Reddy N, Goodman S, Jagasia M, Rezvani K. Management of relapse after allo-SCT for AML and the role of second transplantation. Bone marrow transplantation. 2009;44(12):769–77. doi: 10.1038/bmt.2009.300. [DOI] [PubMed] [Google Scholar]
- 4.Schmid C, Labopin M, Nagler A, Bornhauser M, Finke J, Fassas A, et al. Donor lymphocyte infusion in the treatment of first hematological relapse after allogeneic stem-cell transplantation in adults with acute myeloid leukemia: a retrospective risk factors analysis and comparison with other strategies by the EBMT Acute Leukemia Working Party. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2007;25(31):4938–45. doi: 10.1200/JCO.2007.11.6053. [DOI] [PubMed] [Google Scholar]
- 5.Thanarajasingam G, Kim HT, Cutler C, Ho VT, Koreth J, Alyea EP, et al. Outcome and prognostic factors for patients who relapse after allogeneic hematopoietic stem cell transplantation. Biology of blood and marrow transplantation: journal of the American Society for Blood and Marrow Transplantation. 2013;19(12):1713–8. doi: 10.1016/j.bbmt.2013.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vardiman JW, Harris NL, Brunning RD. The World Health Organization (WHO) classification of the myeloid neoplasms. Blood. 2002;100(7):2292–302. doi: 10.1182/blood-2002-04-1199. [DOI] [PubMed] [Google Scholar]
- 7.Vardiman JW, Thiele J, Arber DA, Brunning RD, Borowitz MJ, Porwit A, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114(5):937–51. doi: 10.1182/blood-2009-03-209262. [DOI] [PubMed] [Google Scholar]
- 8.Grimwade D, Walker H, Oliver F, Wheatley K, Harrison C, Harrison G, et al. The importance of diagnostic cytogenetics on outcome in AML: analysis of 1,612 patients entered into the MRC AML 10 trial. The Medical Research Council Adult and Children’s Leukaemia Working Parties. Blood. 1998;92(7):2322–33. [PubMed] [Google Scholar]
- 9.Warlick ED, Tomblyn M, Cao Q, Defor T, Blazar BR, Macmillan M, et al. Reduced-intensity conditioning followed by related allografts in hematologic malignancies: long-term outcomes most successful in indolent and aggressive non-Hodgkin lymphomas. Biology of blood and marrow transplantation: journal of the American Society for Blood and Marrow Transplantation. 2011;17(7):1025–32. doi: 10.1016/j.bbmt.2010.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brunstein CG, Barker JN, Weisdorf DJ, DeFor TE, Miller JS, Blazar BR, et al. Umbilical cord blood transplantation after nonmyeloablative conditioning: impact on transplantation outcomes in 110 adults with hematologic disease. Blood. 2007;110(8):3064–70. doi: 10.1182/blood-2007-04-067215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brunstein CG, Fuchs EJ, Carter SL, Karanes C, Costa LJ, Wu J, et al. Alternative donor transplantation after reduced intensity conditioning: results of parallel phase 2 trials using partially HLA-mismatched related bone marrow or unrelated double umbilical cord blood grafts. Blood. 2011;118(2):282–8. doi: 10.1182/blood-2011-03-344853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cooley SFB, VM, McKenna D, Luo X, Dusenbery KE, et al. Haploidentical natural killer (NK) cells expanding In vivo after adoptive transfer exhibit hyperfunction that partially overcomes self tolerance and leads to clearance of refractory leukemia. Blood (ASH Annual Meeting Abstracts) 2011 Nov;118:355. [Google Scholar]
- 13.Oran B, Giralt S, Saliba R, Hosing C, Popat U, Khouri I, et al. Allogeneic hematopoietic stem cell transplantation for the treatment of high-risk acute myelogenous leukemia and myelodysplastic syndrome using reduced-intensity conditioning with fludarabine and melphalan. Biology of blood and marrow transplantation: journal of the American Society for Blood and Marrow Transplantation. 2007;13(4):454–62. doi: 10.1016/j.bbmt.2006.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Porter DL, Alyea EP, Antin JH, DeLima M, Estey E, Falkenburg JH, et al. NCI First International Workshop on the Biology, Prevention, and Treatment of Relapse after Allogeneic Hematopoietic Stem Cell Transplantation: Report from the Committee on Treatment of Relapse after Allogeneic Hematopoietic Stem Cell Transplantation. Biology of blood and marrow transplantation: journal of the American Society for Blood and Marrow Transplantation. 2010;16(11):1467–503. doi: 10.1016/j.bbmt.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mortimer J, Blinder MA, Schulman S, Appelbaum FR, Buckner CD, Clift RA, et al. Relapse of acute leukemia after marrow transplantation: natural history and results of subsequent therapy. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 1989;7(1):50–7. doi: 10.1200/JCO.1989.7.1.50. [DOI] [PubMed] [Google Scholar]
- 16.Warlick ED, DeFor T, Blazar BR, Burns L, Verneris MR, Ustun C, et al. Successful remission rates and survival after lymphodepleting chemotherapy and donor lymphocyte infusion for relapsed hematologic malignancies postallogeneic hematopoietic cell transplantation. Biology of blood and marrow transplantation: journal of the American Society for Blood and Marrow Transplantation. 2012;18(3):480–6. doi: 10.1016/j.bbmt.2011.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loren AW, Porter DL. Donor leukocyte infusions for the treatment of relapsed acute leukemia after allogeneic stem cell transplantation. Bone marrow transplantation. 2008;41(5):483–93. doi: 10.1038/sj.bmt.1705898. [DOI] [PubMed] [Google Scholar]
- 18.Levine JE, Braun T, Penza SL, Beatty P, Cornetta K, Martino R, et al. Prospective trial of chemotherapy and donor leukocyte infusions for relapse of advanced myeloid malignancies after allogeneic stem-cell transplantation. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2002;20(2):405–12. doi: 10.1200/JCO.2002.20.2.405. [DOI] [PubMed] [Google Scholar]
- 19.Lee JH, Lee KH, Kim S, Seol M, Kim SH, Kim WK, et al. Combination chemotherapy of intermediate-dose cytarabine, idarubicin, plus etoposide and subsequent mobilized donor leukocyte infusion for relapsed acute leukemia after allogeneic bone marrow transplantation. Leukemia research. 2001;25(4):305–12. doi: 10.1016/s0145-2126(00)00142-9. [DOI] [PubMed] [Google Scholar]
- 20.Choi SJ, Lee JH, Lee JH, Kim S, Seol M, Lee YS, et al. Treatment of relapsed acute myeloid leukemia after allogeneic bone marrow transplantation with chemotherapy followed by G-CSF-primed donor leukocyte infusion: a high incidence of isolated extramedullary relapse. Leukemia. 2004;18(11):1789–97. doi: 10.1038/sj.leu.2403523. [DOI] [PubMed] [Google Scholar]
- 21.Kedmi M, Resnick IB, Dray L, Aker M, Samuel S, Gesundheit B, et al. A retrospective review of the outcome after second or subsequent allogeneic transplantation. Biology of blood and marrow transplantation: journal of the American Society for Blood and Marrow Transplantation. 2009;15(4):483–9. doi: 10.1016/j.bbmt.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 22.Kolb HJ, Schattenberg A, Goldman JM, Hertenstein B, Jacobsen N, Arcese W, et al. Graft-versus-leukemia effect of donor lymphocyte transfusions in marrow grafted patients. Blood. 1995;86(5):2041–50. [PubMed] [Google Scholar]
- 23.Eapen M, Giralt SA, Horowitz MM, Klein JP, Wagner JE, Zhang MJ, et al. Second transplant for acute and chronic leukemia relapsing after first HLA-identical sibling transplant. Bone marrow transplantation. 2004;34(8):721–7. doi: 10.1038/sj.bmt.1704645. [DOI] [PubMed] [Google Scholar]
- 24.Vago L, Perna SK, Zanussi M, Mazzi B, Barlassina C, Stanghellini MT, et al. Loss of mismatched HLA in leukemia after stem-cell transplantation. The New England journal of medicine. 2009;361(5):478–88. doi: 10.1056/NEJMoa0811036. [DOI] [PubMed] [Google Scholar]