Abstract
Mycophenolate mofetil (MMF) is frequently used in hematopoietic cell transplantation (HCT) for graft-versus-host disease (GVHD) prophylaxis and to facilitate engraftment. We previously reported that a higher level of mycophenolic acid can be achieved with an MMF dose of 3 g/day as compared to 2g/day. Here, we retrospectively compared clinical outcomes of reduced intensity conditioning (RIC) double umbilical cord blood (dUCB) HCT recipients receiving cyclosporine A with MMF 2g (n=93) vs. 3g (n=175) daily. Multiple regression analysis adjusted for ATG in the conditioning revealed that MMF 3g/day led to a 49% relative risk reduction in grade II–IV acute GVHD rate (RR=0.51, 95%CI 0.36–0.72; p<0.01). However, the higher MMF dose was not protective for chronic GVHD. Additionally, MMF dose was not an independent predictor of neutrophil engraftment, treatment-related mortality at 6 months, or 2-year post-transplant disease relapse, disease-free survival, or overall survival. Higher MMF dose did not increase risk of infectious complications and infection-related mortality was similar for both MMF doses. Our data indicate that MMF 3g/day reduces the risk of acute GVHD without affecting other clinical outcomes and should be used for GVHD prophylaxis after RIC dUCBT.
Keywords: Transplantation, MMF, GVHD, RIC, UCB
INTRODUCTION
Double umbilical cord blood (dUCB) hematopoietic cell transplantation (HCT) in the myeloablative and reduced intensity conditioning (RIC) settings has extended the use of UCB grafts to adults and large adolescents who would not have a suitable single-unit UCB graft.1–3 Although engraftment remains delayed and less complete among UCB recipients than adult-donor recipients,4 it is similar among recipients of single and double UCB grafts.5, 6 However, we found that the risk of grade II–IV, but not grade III–IV acute graft-vs.-host disease (GVHD) is higher among double UCB recipients than single UCB recipients.7 We and others use mycophenolate mofetil (MMF) as part of the immune suppression regimen in HCT.2, 3, 7–12 Our group previously demonstrated that a low unbound mycophenolic acid (MPA) area under the curve (AUC), which is the active metabolite of MMF, is associated with higher rates of graft failure and grade II–IV acute GVHD in RIC UCB, matched sibling, and adult unrelated donor transplantation.13 In addition, a report of adult unrelated donor HCT using a fludarabine/total body irradiation (TBI)-based non-myeloablative conditioning regimen suggests superior engraftment with a higher MPA AUC.9 On the basis of these observations, we modified our UCB HCT protocols and increased the MMF dose from 2 g to 3 g per day. In pharmacokinetic studies, we showed that MMF 3g/day, administered either as 1.5 g twice daily or 1 g thrice daily, achieved the unbound 24-hour cumulative MPA target AUC (0.600 µg*h/ml) in over 87% of patients, in contrast to MMF 2g/day (1 g twice daily) where less than half of patients achieved the target AUC.13, 14 Here, we report the outcomes associated with use of MMF 3g/day in dUCB HCT.
METHODS
Patients
Eligible patients included adults with hematologic malignancy who received a RIC double UCB HCT at the University of Minnesota between 2000–2012. Our group previously reported 110 of these patients.1, 3 All patients were treated in clinical protocols approved by the Institutional Review Board of the University of Minnesota, and either the patient or his/her legal guardian provided written informed consent in accordance with the Declaration of Helsinki prior of proceeding with transplantation.
Immune suppression regimen
All patients received immunosuppressive therapy consisting of MMF and cyclosporine A (CSA). MMF was administered from days −3 to +30 or 7 days after neutrophil engraftment, whichever was later, in the absence of acute GVHD. MMF was administered 2 g/day twice daily (2000–2005) or 3 g/day (2006–2012) in 2 or 3 divided doses. While research-related MMF/MPA pharmacokinetics data were obtained in a subset of patients, as previously reported,13, 14 MPA AUC was not used to personalize MMF dosing. CSA was administered from day −3 to day +180 with a target trough level between 200 and 400 ng/mL and, in the absence of GVHD, tapered by day +180.15
Treatment
UCB graft selection criteria, conditioning regimens, and supportive care have been previously reported.1–3 In summary, a minimum of 4/6 HLA loci were matched to the patient; HLA-A and HLA-B were matched at the antigen level, and HLA-DRB1 was matched at the allele level.3 The two UCB units were HLA-matched to each other at a minimum of 4/6 HLA loci, but not necessarily at the same loci as the patient. Target selection for cryopreserved total nucleated cell (TNC) dose for the two UCB units ranged from 2 to 3.5 × 107 TNC/kg thereafter. The non-myeloablative regimen consisted of fludarabine 200 mg/m2, CY 50 mg/kg, and a single fraction of TBI 200 cGy.3 Equine antithymocyte globulin (ATG; 90 mg/kg) was included in the preparative regimen for those patients who received no immunosuppressive multiagent chemotherapy within the prior 3 months or with no prior autologous transplant; since 2005 if autologous transplant within 12 months of the allograft.1, 3 Anti-infectious prophylaxis included fluoroquinolone for bacterial infections, either fluconazole or voriconazole for fungal infections, acyclovir for viral infections, and trimethoprim-sulfamethoxazole or pentamidine for Pneumocystis jiroveci infection.3 Granulocyte–colony stimulating factor (G-CSF; 5 µg/kg/day) was administered to all patients from day 0 or +1 until absolute neutrophil count (ANC) > 2.5 × 109 /L for 2 consecutive days.1, 3
Definitions and endpoints
Data on baseline patient and disease characteristics, transplant-related factors and clinical outcome measures were prospectively collected and recorded by the University of Minnesota Blood and Marrow Transplant. Study endpoints included cumulative incidences of neutrophil engraftment by day +42 (defined as ANC > 0.5 × 109 /L for 3 consecutive days), non-relapse mortality (NRM) at 6 months, and relapse/progression at 2 years, as well as the probabilities of disease-free survival (DFS; defined as being alive and with no evidence of disease relapse/progression) and overall survival (OS). Additional endpoints included the cumulative incidences of acute GVHD at day +100 and chronic GVHD at +2 years. As our study included patients treated prior to the adoption of the consensus chronic GVHD criteria,16 acute GVHD was defined as any signs or symptoms of GVHD occurring by day +100 according to published criteria.17 Chronic GVHD was defined as any signs or symptoms of GVHD occurring after day +100. Frequency and density of infections was studied within specific post-transplant time intervals of days 0 to +45, days +46 to +180, and days +181 to +365. The day of each infectious episode was calculated from the day of transplant (day 0). Pathogenic organisms were categorized based on genus into bacterial, fungal, or viral groups. Neutropenic fever events without identifiable infectious source were not included. An infectious episode was defined as any infection requiring treatment that was identified by culture, cytology, histology, antigenemia, or polymerase chain reaction (PCR). No microbiologic confirmation was required for documentation of dermatomal varicella-zoster viral (VZV) infectious episode. Human herpesvirus 6 (HHV6) PCR testing became routinely available at our center in 2006; therefore, cases of HHV6 infection were not included in this analysis to avoid confounding bias with MMF dosing. Cytology findings of unspeciated fungal elements were acceptable for documentation of probable invasive fungal infections (IFI) if compatible with the clinical scenario. Specific time frames were used to separate one infectious episode from another: 7 days was required for bacteria (except 31 days for C. difficile), 90 days for mold (14 days for yeast), and 60 days for CMV and HSV (14 days for other viruses).18 On the basis of the affected site, infections were categorized as blood stream, central nervous system (CNS), gastrointestinal (GI), intra-abdominal, urinary tract (UTI), upper respiratory tract, lower respiratory tract, skin and soft tissue, and other infections or viral reactivation. Sex and HLA matching between UCB and patient was studied considering the worst matched of the two UCB units. Disease risk at the time of HCT was classified into standard risk or high risk on the basis of the ASBMT Request for Information (RFI) 2006 risk scoring schema (http://www.asbmt.org). Standard-risk disease included acute leukemia in first or second complete remission, chronic myeloid leukemia (CML) in first chronic phase, Hodgkin or non-Hodgkin lymphoma in complete or partial chemotherapy-sensitive remission, chronic lymphocytic leukemia (CLL) in first remission; all other disease states were determined to be high risk at the time of transplantation. Patients were assessed for the HCT comorbidity index (HCT-CI) as previously reported.19
Statistical Analysis
Statistical comparisons for categorical factors were completed with the chi-square test. Continuous factors across two MMF doses were compared by the general Wilcoxon test for non-parametric data. Kaplan-Meier curves were used to estimate the probability of DFS or OS using Greenwood’s method to calculate the 95% confidence intervals.20 The log-rank test was used to complete the comparisons. Cox regression was used to examine the independent effect of MMF dose on DFS and OS through 2 years post-transplant.21 Factors considered in the regression models included highest mismatch for HLA disparity considering the worst matched of the two UCB units (4/6 versus 5/6 versus 6/6), age (18–34 versus 35–59 versus 60+), disease risk (standard versus high risk), gender (male versus female), gender match (female donor to male recipient), Karnofsky performance status at baseline (60–80% versus 90–100%), HCT-CI (0 versus 1–2 versus ≥3), recipient CMV serostatus (positive versus negative). Analysis by MMF dose cohort was tested for any violations in the proportional hazards assumption. Cumulative incidence treating non-event deaths as a competing risk was used to estimate the probability of neutrophil engraftment, relapse, and acute and chronic GVHD. Cumulative incidence treating relapse as a competing risk was used to estimate NRM.22 Fine and Gray regression analyses were used to look at the MMF dose cohort for the endpoints of engraftment, relapse, NRM, and GVHD.23 Backward selection was used to build prognostic factor models for all endpoints considering a p-value of < 0.10. A p-value of ≤ 0.05 was considered significant for remaining in the model; however, MMF dose was included in all models regardless of significance. The adjusted cumulative incidence curves were estimated for acute GVHD based on risk factors significant in the regression model.24, 25 Incorporating multiple infections per patient, the rates of infections were estimated by the infection density per 1000 patient days. We used specific post-transplant time intervals (days 0–45, days 46–180 and days 181–365) to study the effect of MMF dose on frequency of bacterial, fungal, and viral infections. Comparisons were completed with the Mantel-Haenszel test for person-years data.
RESULTS
Patient Characteristics
We identified 268 patients with hematologic malignancy who received RIC dUCB HCT at the University of Minnesota between 2000–2012 (Table 1). Nearly twice as many patients received MMF 3 g/day (n= 175) than MMF 2 g/day (n= 93). Median age at transplant was 53 years (range, 18–72). As defined in this retrospective study design, the “year of transplant” variable was associated with MMF dose. Patients receiving MMF 3g/day received a higher MMF dose per kilogram body weight (mg/kg) and better HLA-matched grafts, which is consistent with the larger inventory of UCB units in recent years. Otherwise, the two groups were similar regarding patient and disease characteristics, proportion of CMV seropositivity, HCT-CI score, use of ATG as part of the preparative regimen, and total infused CD34+ cell dose.
Table 1.
Patient characteristics
| Variable | MMF 2mg n=93 N (%) |
MMF 3mg n=175 N (%) |
P-value* | |
|---|---|---|---|---|
| Age (years) | Median (range) | 52 (21–69) | 54 (18–72) | 0.18 |
| Male | 58 (62) | 105 (60) | 0.71 | |
| Year of HCT | <0.001 | |||
| 2000–2005 | 92 (99) | 4 (2) | ||
| 2006–2012 | 1 (1) | 171 (98) | ||
| HLA disparity (worst match) | <0.001 | |||
| 4/6 | 73 (78) | 82 (47) | ||
| 5/6 | 18 (19) | 79 (45) | ||
| 6/6 | 2 (2) | 14 (8) | ||
| ATG in conditioning | 29 (31) | 57 (33) | 0.82 | |
| Diagnosis | 0.24 | |||
| AML | 31 (33) | 73 (42) | ||
| MDS | 11 (12) | 31 (18) | ||
| CML | 6 (7) | 3 (2) | ||
| ALL | 9 (10) | 13 (7) | ||
| Lymphoma | 25 (27) | 42 (24) | ||
| CLL | 6 (7) | 7 (4) | ||
| Other† | 5 (5) | 6 (4) | ||
| Disease risk | 0.23 | |||
| Standard | 39 (42) | 87 (50) | ||
| High | 54 (58) | 88 (50) | ||
| HCT-CI | 0.60 | |||
| 0 | 20 (22) | 47 (27) | ||
| 1–2 | 25 (27) | 41 (23) | ||
| ≥ 3 | 42 (45) | 87 (50) | ||
| Missing | 6 (7) | 0 | ||
| CMV seropositive | 49 (53) | 109 (62) | 0.13 | |
| Total CD34 (×106/kg) | Median (range) | 0.5 (0.1–1.7) | 0.5 (0.1–3.5) | 0.24 |
| Weight (kg) | Median (range) | 78.2 (52.7–134.0) | 80.4 (43.7–142.3) | 0.47 |
| Body Mass Index | Median (range) | 26.7 (19.6–39.6) | 27.5 (17.2–45.0) | 0.20 |
| MMF (mg/kg) | Median (range) | 2.56 (1.49–3.80) | 3.73 (2.11–6.86) | <0.01 |
| 1st Quartile (<2.7) | 55 (59) | 32 (18) | <0.01 | |
| 2ndQuartile (2.7–3.2) | 29 (31) | 32 (18) | ||
| 3rd Quartile (3.2–3.9) | 9 (10) | 51 (29) | ||
| 4th Quartile (>3.9) | 0 | 60 (34) |
P-value for between-treatment comparisons. Continuous variables were analyzed by general Wilcoxon test. Categorical variables were analyzed by chi-square.
Other includes diagnoses of plasma cell disorders and biphenotypic leukemia
Higher MMF dose reduces the risk of acute GVHD
After adjusting for use of ATG, the cumulative incidence of grade II–IV acute GVHD by day +100 was significantly lower in those receiving MMF 3g/day (43% vs. 63%; p<0.01; Figure 1), and grade III–IV acute GVHD showed a trend towards being significantly lower in those receiving 3g/day (14% vs. 23%; p=0.06). Among patients who developed grade II–IV acute GVHD, the proportion of liver involvement was significantly higher in those receiving MMF 2g/day (12% vs. 34%; p<0.01); however, other specific organ or multi-organ involvement with acute GVHD was similar for both MMF doses (Figure 2). In multivariable analysis, after adjusting for ATG use in conditioning regimen, MMF 3g/day was associated with 49% relative risk reduction in grade II–IV acute GVHD (RR 0.51, 95% CI 0.36–0.72; p<0.01; Table 2).
Figure 1.
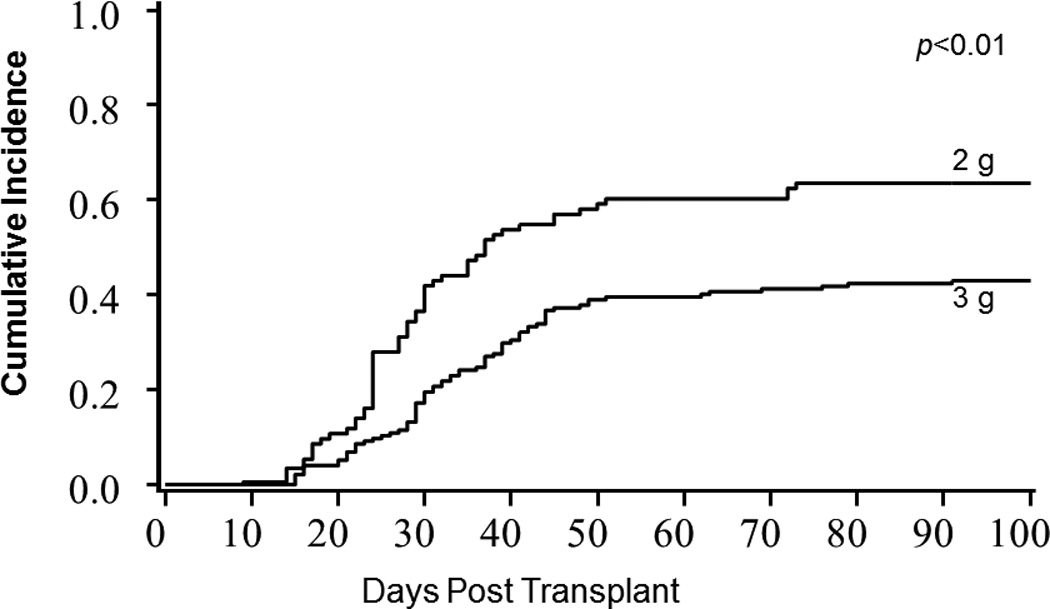
Grade II–IV acute GVHD by MMF Dose
Figure 2.
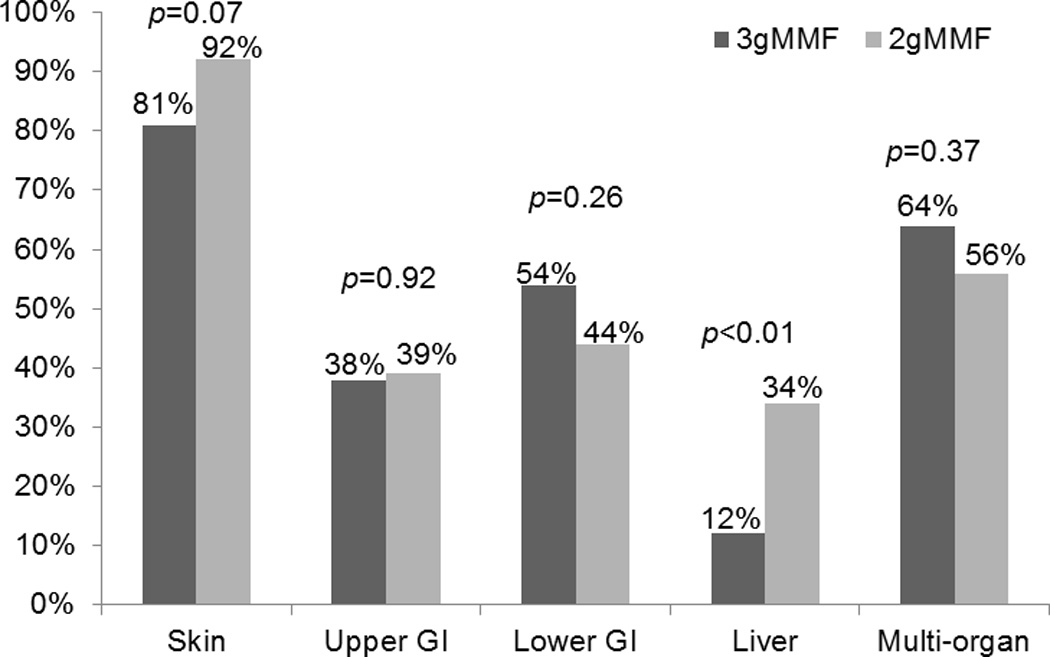
Organ involvement with grade II–IV aGVHD
Table 2.
Grade II–IV Acute GvHD by Day 100
| Variable | Cumulative Incidence | Multivariable | ||||||
|---|---|---|---|---|---|---|---|---|
| N | Estimate (%) |
95% CI | P-value | RR | 95% CI | P-value | ||
| MMF | <0.01 | |||||||
| 2g | 93 | 63 | 52–75 | 1.0 | ||||
| 3g | 175 | 43 | 35–51 | 0.51 | 0.36–0.72 | <0.01 | ||
| Age | 0.01 | -- | -- | -- | ||||
| 18–34 years | 26 | 46 | 26–66 | |||||
| 35–59 years | 176 | 57 | 49–65 | |||||
| 60+ years | 66 | 33 | 22–45 | |||||
| Gender | 0.85 | -- | -- | -- | ||||
| Male | 163 | 50 | 42–59 | |||||
| Female | 105 | 50 | 39–60 | |||||
| Year of HCT | <0.01 | -- | -- | -- | ||||
| 2000–2005 | 96 | 64 | 52–75 | |||||
| 2006–2012 | 172 | 42 | 33–50 | |||||
| HLA disparity | 0.89 | -- | -- | -- | ||||
| 4/6 | 155 | 49 | 41–57 | |||||
| 5/6 | 97 | 52 | 41–62 | |||||
| 6/6 | 16 | 50 | 24–76 | |||||
| Disease risk | 0.65 | -- | -- | -- | ||||
| Standard risk | 126 | 49 | 40–59 | |||||
| High-risk | 142 | 51 | 42–60 | |||||
| HCT-CI | 0.82 | -- | -- | -- | ||||
| 0 | 67 | 54 | 41–67 | |||||
| 1–2 | 66 | 50 | 37–63 | |||||
| ≥3 | 129 | 47 | 37–56 | |||||
| Female donor to male recipient | 0.94 | -- | -- | -- | ||||
| Yes | 141 | 50 | 41–59 | |||||
| No | 127 | 50 | 40–59 | |||||
| CMV serostatus | 0.78 | -- | -- | -- | ||||
| Positive | 110 | 51 | 41–61 | |||||
| Negative | 158 | 49 | 41–58 | |||||
| ATG | 0.06 | |||||||
| No | 182 | 54 | 46–62 | 1.0 | ||||
| Yes | 86 | 41 | 30–52 | 0.60 | 0.41–0.88 | 0.01 | ||
Cumulative incidence of chronic GVHD was not significantly different between the MMF doses (20% vs. 27%, p=0.17; Table 3). In multivariable analysis, after adjusting for gender and female donor use for male recipient, MMF dose had no significant influence on chronic GVHD (RR=0.69 for MMF 3g/day, 95% CI 0.41–1.15; p=0.15).
Table 3.
Clinical Outcomes by MMF Dose
| Variable | Univariable | Multivariable | ||||||
|---|---|---|---|---|---|---|---|---|
| N | Estimate (%) |
95% CI | P-value | RR | 95% CI | P-value | ||
| Neutrophil Engraftment | ||||||||
| MMF | 0.96 | |||||||
| 2g | 93 | 94 | 91–97 | 1.0 | ||||
| 3g | 175 | 93 | 88–98 | 0.96 | 0.71–1.28 | 0.76 | ||
| HLA disparity | 0.20 | |||||||
| 4/6 | 155 | 93 | 89–97 | 1.0 | ||||
| 5/6 | 97 | 95 | 91–99 | 1.18 | 0.89–1.57 | 0.25 | ||
| 6/6 | 16 | 100 | 1.64 | 0.91–2.97 | 0.10 | |||
| Age | 0.02 | |||||||
| <34 | 26 | 100 | 1.0 | |||||
| 35–59 | 176 | 95 | 92–98 | 1.07 | 0.80–1.43 | 0.25 | ||
| 60+ | 66 | 89 | 81–97 | 0.69 | 0.49–0.96 | 0.03 | ||
| CD34 dose | 0.02 | |||||||
| <3.5×107 | 64 | 89 | 81–97 | 1.0 | ||||
| ≥3.5×107 | 204 | 95 | 92–98 | 1.40 | 1.03–1.90 | 0.03 | ||
| Platelet Engraftment (20K) | ||||||||
| MMF | 0.16 | |||||||
| 2g | 93 | 68 | 56–80 | 1.0 | ||||
| 3g | 175 | 75 | 66–84 | 1.26 | 0.93–1.70 | 0.14 | ||
| HLA disparity | 0.03 | -- | -- | -- | ||||
| 4/6 | 155 | 71 | 62–80 | |||||
| 5/6 | 97 | 75 | 63–87 | |||||
| 6/6 | 16 | 75 | 46–100 | |||||
| HCT-CI | 0.04 | |||||||
| None | 67 | 82 | 64–87 | 1.0 | ||||
| 1–2 | 66 | 76 | 62–80 | 0.82 | 0.55–1.22 | 0.33 | ||
| 3+ | 129 | 67 | 57–78 | 0.67 | 0.47–0.94 | 0.02 | ||
| CD34 dose | <0.01 | |||||||
| <3.5–107 | 64 | 58 | 44–72 | 1.0 | ||||
| ≥3.5×107 | 204 | 77 | 69–86 | 1.81 | 1.29–2.55 | <0.01 | ||
| Chronic GVHD | ||||||||
| MMF | 0.17 | |||||||
| 2g | 93 | 27 | 17–36 | 1.0 | ||||
| 3g | 175 | 20 | 14–26 | 0.69 | 0.41–1.15 | 0.15 | ||
| Gender | 0.14 | |||||||
| Male | 163 | 26 | 19–33 | 1.0 | ||||
| Female | 105 | 17 | 10–25 | 0.41 | 0.28–0.84 | 0.01 | ||
| Female donor to male recipient | 0.93 | |||||||
| No | 141 | 22 | 15–29 | 1.0 | ||||
| Yes | 127 | 23 | 15–31 | 0.54 | 0.28–1.04 | 0.07 | ||
| NRM | ||||||||
| MMF | 0.74 | |||||||
| 2g | 93 | 15 | 8–22 | 1.0 | ||||
| 3g | 175 | 14 | 9–19 | 0.93 | 0.48–1.81 | 0.83 | ||
| Disease risk | 0.02 | |||||||
| Standard | 126 | 9 | 4–14 | 1.0 | ||||
| High | 142 | 19 | 13–26 | 2.28 | 1.13–4.61 | 0.02 | ||
| ATG | 0.07 | -- | -- | -- | ||||
| No | 182 | 12 | 7–16 | |||||
| Yes | 86 | 20 | 11–28 | |||||
| Relapse | ||||||||
| MMF | 0.78 | |||||||
| 2g | 93 | 40 | 29–51 | 1.0 | ||||
| 3g | 175 | 43 | 35–51 | 1.14 | 0.77–1.69 | 0.52 | ||
| Female donor to male recipient | 0.16 | |||||||
| No | 141 | 38 | 29–46 | 1.0 | ||||
| Yes | 127 | 46 | 36–55 | 1.33 | 0.92–1.92 | 0.13 | ||
| ATG | 0.05 | |||||||
| No | 182 | 47 | 39–55 | 1.0 | ||||
| Yes | 86 | 31 | 20–41 | 0.56 | 0.36–0.87 | 0.01 | ||
| DFS | ||||||||
| MMF | 0.88 | |||||||
| 2g | 93 | 40 | 30–50 | 1.0* | ||||
| 3g | 175 | 37 | 30–44 | 1.05* | 0.77–1.43 | 0.74 | ||
| CD34 dose | 0.02 | |||||||
| <3.5×107 | 64 | 29 | 19–41 | 1.0* | ||||
| ≥3.5×107 | 204 | 41 | 34–48 | 0.69* | 0.49–0.96 | 0.03 | ||
| OS | ||||||||
| MMF | 0.85 | |||||||
| 2g | 93 | 62 | 52–71 | 1.0$ | ||||
| 3g | 175 | 61 | 53–67 | 1.03$ | 0.72–1.47 | 0.87 | ||
| CD34 dose | 0.03 | |||||||
| <3.5×107 | 64 | 50 | 37–61 | 1.0$ | ||||
| ≥3.5×107 | 204 | 65 | 58–71 | 0.70$ | 0.48–1.02 | 0.07 | ||
Denotes relative risk of death/relapse
Denotes relative risk of death
MMF dose does not influence hematopoietic recovery
Cumulative incidences of neutrophil recovery were 93% for MMF 3g/day and 94% for MMF 2 g/day. Median time to neutrophil engraftment was similar for both MMF doses (14 days 3g/day and 12 days 2g/day; p=0.96; Figure 3). In univariable analysis, age and infused CD34 dose were the factors associated with neutrophil engraftment by day 42 (Table 3). In multivariable analysis, MMF dose was not an independent predictor of neutrophil engraftment (MMF 3g/day, RR = 0.96, 95% CI 0.71–1.28; p=0.76). However, age (≥60 years, RR=0.69, 95% CI 0.49–0.96; p=0.03) was associated with poor engraftment while higher total infused CD34 dose (≥3.5×107, RR=1.40, 95% CI 1.03–1.90; p=0.03) was associated with more frequent engraftment. Cumulative incidences of platelet recovery were 75% for MMF 3g/day and 68% for MMF 2g/day. Median time to platelet recovery was similar for both MMF doses (48 days MMF 3g/day and 58 days MMF 2g/day; p=0.16). However, a comorbidity score of ≥3 was a predictor of poor platelet engraftment (RR=0.67, 95% CI 0.47–0.94; p=0.02) while higher total infused CD34 dose (≥3.5×107, RR=1.81, 95% CI 1.29–2.55; p<0.01) was associated with more frequent engraftment.
Figure 3.
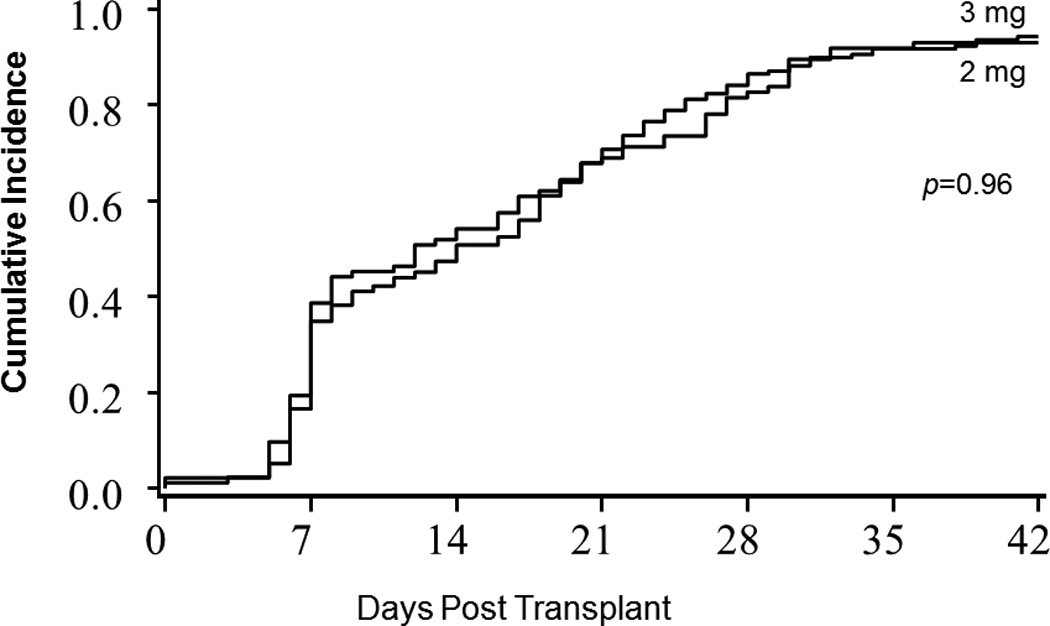
Neutrophil Engraftment by MMF Dose
Higher MMF dose does not increase risk of infectious complications
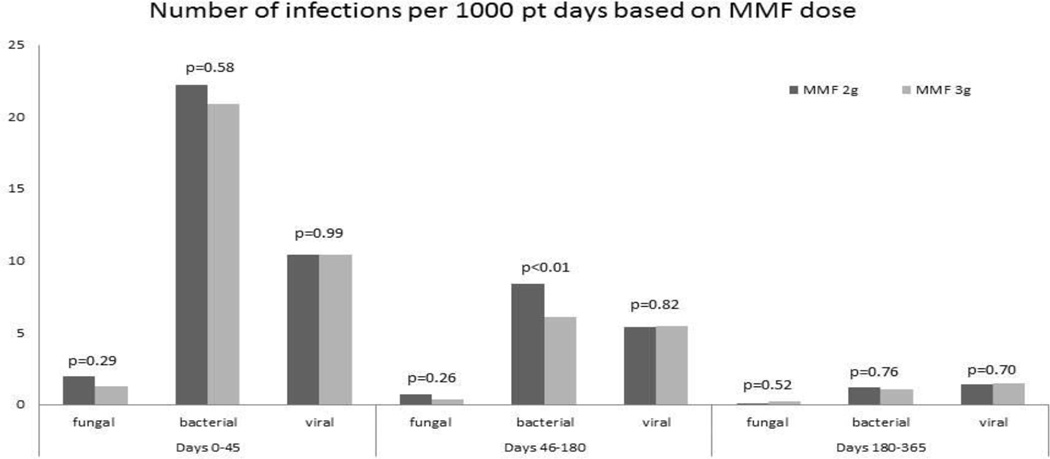
Overall, there were 402 bacterial infection events. The most commonly isolated bacterial pathogen was staphylococcus (40%). Other bacterial pathogens isolated less frequently were enterococcus (21%), Clostridium difficile (15%), mycobacterium (4%), and others (total 20%). Fungal infections were relatively rare (n= 34). Aspergillosis was the most frequent (38%) fungal infection, followed by candida and other yeasts (29%), zygomycosis (21%), hyalohyphomycosis (9%), and unspecified IFI (3%). Viral infectious events (n=313) included CMV (43%), BK virus (13%), EBV (9%), adenovirus (8%), VZV (7%), HSV (7%), and others (13%).To assess the effect of MMF dosing on risk of infection, we used the infection density function per 1000 patient days, which takes into account multiple infections in an individual patient within specific post-transplant time intervals. MMF dose had no impact on bacterial infections at early (days 0–45; 13.65 vs. 18.40; p=0.05) or late (days 181–365; 0.74 vs. 1.49; p=0.03) post-transplant time periods. However, MMF 3g/day was associated with a lower density of bacterial infections (4.63 vs. 9.44; p<0.01) and total serious infections (all genus types combined, 10.14 vs. 15.63; p<0.01) between post-transplant days 46–180 (Figure 4). In addition, the infection density rates of both fungal and viral infections were similar for both MMF doses at all post-transplant time intervals. Cumulative incidence of infection-related death at 1 year was similar for both MMF doses (12% for 3g/day vs. 14% for 2g/day; p=0.59).
Figure 4.
Infection Density by MMF dose
Higher MMF dose does not influence risk of relapse or survival
Cumulative incidence of NRM at 6 months was similar for both MMF doses (14% for 3g/day vs. 15% for 2g/day; p=0.74). In univariable analysis, high-risk disease resulted in increased risk of NRM (Table 3). In multivariable analysis, after adjusting for disease risk and ATG use in conditioning, the higher MMF dose had no impact on NRM (RR=0.93 for MMF 3g/day, 95% CI 0.48–1.81; p=0.83). Similarly there were no differences in relapse rates at 2 years by MMF dose (43% for 3g/day vs. 40% for 2g/day; p=0.78). In univariable analysis, ATG use in conditioning was associated with a decreased rate of relapse. In multivariable analysis, after adjusting for use of ATG in the conditioning regimen, there was no association between MMF dose and relapse (RR=1.14 for MMF 3g/day, 95% CI 0.77–1.69; p=0.52). The probability of DFS at 2 years was 37% for 3g/day versus 40% for 2g/day (p=0.88). In univariate analysis, low (<3.5×107/kg) CD34 cell dose was the only factor associated with inferior DFS. In Cox regression analysis, low CD34 cell dose remained predictive for treatment failure, but MMF 3g/day dose had no impact on it (RR=1.05, 95% CI 0.77–1.43; p=0.74). Survival of all patients at 2 years post-transplant was 61% for MMF 3g/day and 62% for MMF 2g/day (p=0.85). In univariable analysis, better survival was observed only among patients receiving total ≥3.5×107/kg CD34 cell dose. In Cox regression analysis, survival was not significantly influenced by total infused CD34 cell dose or MMF dose (RR=1.03 for 3gMMF, 95% CI 0.72–1.47; p=0.87). Primary causes of death at 2 years were similar for 3g and 2g MMF doses, with the predominant causes of mortality being malignancy relapse (65% vs. 60%), followed by infection (16% vs. 15%), organ toxicity or failure (6% vs. 8%), bleeding (1% vs. 4%), graft failure (1% vs. 0%), and other causes (11% vs. 13%).
DISCUSSION
We compared the clinical outcomes of patients with hematological malignancies undergoing RIC dUCB HCT who received an immunosuppressive regimen that included either MMF 3g/day or MMF 2g/day in combination with CSA. MMF dose had no significant effect on hematopoietic recovery, risk of infectious complications, relapse, or mortality, but the higher dose of MMF resulted in a significantly lower incidence of grade II–IV acute GVHD. In addition, we observed a non-statistically significant lower incidence of grade III–IV acute GVHD with higher dose of MMF; however, given the overall low incidence of grade III–IV acute GVHD in UCB transplantation, a larger patient cohort will be required to better study this association. This finding extends our previous reports showing that MMF 3g/day not only increases the proportion of patients achieving therapeutic MPA target AUC, but also lowers the risk of acute GVHD when adequate MPA AUC is achieved.13, 14 This observation was recently reproduced by others as well.12, 26 The Seattle group previously reported that administration of higher dose MMF had no effect on the risk of GVHD;10 however, this group recently reported that higher therapeutic MPA AUC protected recipients of adult unrelated donor RIC transplantation from acute GVHD.12 While MPA AUC testing is clinically available, we used uniform dosing and did not guide MMF dosing by MPA AUC. In a smaller number of patients, Harnicar et al. presented data showing a correlation between higher MPA levels and lower risk of acute GVHD in dUCB HCT with MMF and CSA based immunosuppression.26 Notably, in our series the reduction in acute GVHD was limited to grade II and liver involvement, with no effect on severe acute or chronic GVHD. The data from our group and others demonstrate that a higher dose MMF results in better exposure to MPA and reduces acute GVHD, and importantly does not compromise engraftment, infection risks, or the anti-neoplastic effects of the HCT. Despite the use of MMF after haploidentical donor transplantation,27, 28 we recommend caution in extrapolating our MMF data as its effect has not been yet studied in this setting.
As our study included patients treated prior to the adoption of the consensus criteria,16 we used clinical criteria to differentiate acute GVHD from chronic GVHD.17 While there could have been some additional earlier cases of late onset acute and overlap presentations of chronic GVHD recognized in the 2g MMF cohort, the robust 18% absolute and 42% relative risk reduction in the risk of GVHD would be unlikely to significantly change our conclusions. Immunosuppressive therapy in our study consisted of MMF and CSA; therefore, the proposed MMF 3g/day dose examined in this study cannot be generalized to MMF when used in combination with tacrolimus because of underlying pharmacokinetic differences.29
In our study, patients receiving MMF 3g/day received better matched UCB grafts, possibly reflecting a larger inventory of UCB and our ability to find better matched grafts for our patients in recent years. However, previous reports by our group and others failed to demonstrate a significant impact of HLA matching on GVHD rates after UCB HCT.7, 30 In contrast, one recent study reported a reduction in the risk of severe GVHD after dUCB HCT for patients with better allele-level HLA-matching of the predominant UCB unit.31 However, in our sample, the effect of allele-level typing on the outcomes of dUCB HCT did not confirm this association.32 Thus, in the complex microenvironment after dUCB HCT, the effect of HLA-matching on the risk of acute GVHD remains uncertain.
Our findings that a higher dose of MMF did not affect hematopoietic engraftment or infections provide clinically useful information. While MMF can cause leukopenia and, therefore, could potentially have adverse impact on hematopoietic recovery, neutrophil engraftment was not impaired by higher MMF dose as originally reported by our group13 and others.9, 10 The data from Maris et al. showed an improvement on sustained engraftment from 85% in their historical control (MMF 15mg/kg twice daily) to 95% with a higher MMF dose (MMF 15mg/kg thrice daily).10 The engraftment rate in our historical controls was 93% in contrast to 90% with the higher 3g/day dose of MMF. Thus, even if engraftment were enhanced by a higher MMF dose, which it was not, it would take a substantially larger sample size to demonstrate a statistically significant improvement from this higher baseline success rate. In addition, we observed no adverse effect of MMF 3g/day on risk of infections or infection-related death post-HCT. In contrast, Maris et al. found a higher risk of infections early post-HCT in patients receiving a higher MMF dose.10 Improvements in supportive care, infectious prophylaxis, and treatment may explain, at least in part, the similar infection risk between the two MMF dose levels.
Despite the lower rate of acute GVHD in patients receiving MMF 3g/day, we observed no differences in NRM between the MMF dose levels. This was not unexpected because the effect was largely limited to the risk of grade II GVHD, and not grades III–IV acute GVHD. In a previous study, grade III–IV acute GVHD has been associated with higher treatment failure after UCB HCT.3 Data from McDermott et al. suggests that identification of patients with inadequate MPA levels and adjusting the dose would also reduce the risk of severe GVHD and NRM.12 While we do not have specific data on, for example, gastrointestinal side effects associated with a higher MMF dose, myelosuppression as measured by time to engraftment was a possible surrogate measure that was not adversely affected by MMF 3g/day.
Similarly, a higher MMF dose did not affect disease relapse after HCT. While the no ATG group had a higher incidence of malignancy relapse after transplantation, this association was expected with omission of ATG in high-risk disease cases per study protocol. Higher infused total CD34+ dose was associated with improved neutrophil and platelet engraftment and lower risk of treatment failure. However, a higher MMF dose was not associated with treatment failure or mortality after HCT.
In conclusion, our study supports the use of MMF 3g/day in the context of RIC dUCB transplantation. Although pharmacokinetic monitoring of MPA might be useful for individualized MMF dosing, given that MMF 3g was well tolerated, the cost-benefit of such intervention needs to be carefully considered. However, if we were to pursue MMF doses in the higher end of the spectrum of our study (≥40 mg/kg), we would need to closely monitor pharmacokinetics and adverse effects, and such a study should only be undertaken in the context of a clinical trial.
Acknowledgments
This work was supported in part by grants from the National Cancer Institute P01 CA65493 (C.G.B, J.E.W, T.E.D), the Children’s Cancer Research Fund (J.E.W., T.E.D), Leukemia and Lymphoma Society Scholar in Clinical Research Award, grant R6029-07 (C.G.B.).
We would like to acknowledge Michael Franklin, MS, for assistance in editing this manuscript.
Footnotes
Authorship: N.B performed research, analyzed data, and wrote the paper. C.G.B. designed research, performed research, analyzed data, and wrote the paper. J.R. performed research and wrote the paper. T.E.D. analyzed data and wrote the paper. K. E. performed research. A.L., S.H., M.A., M.L.M., D.J.W., P. J. and J.E.W performed research and wrote the paper.
REFERENCES
- 1.Barker JN, Weisdorf DJ, DeFor TE, Blazar BR, Miller JS, Wagner JE. Rapid and complete donor chimerism in adult recipients of unrelated donor umbilical cord blood transplantation after reduced-intensity conditioning. Blood. 2003;102(5):1915–1919. doi: 10.1182/blood-2002-11-3337. [DOI] [PubMed] [Google Scholar]
- 2.Barker JN, Weisdorf DJ, DeFor TE, Blazar BR, McGlave PB, Miller JS, et al. Transplantation of 2 partially HLA-matched umbilical cord blood units to enhance engraftment in adults with hematologic malignancy. Blood. 2005;105(3):1343–1347. doi: 10.1182/blood-2004-07-2717. [DOI] [PubMed] [Google Scholar]
- 3.Brunstein CG, Barker JN, Weisdorf DJ, DeFor TE, Miller JS, Blazar BR, et al. Umbilical cord blood transplantation after nonmyeloablative conditioning: impact on transplantation outcomes in 110 adults with hematologic disease. Blood. 2007;110(8):3064–3070. doi: 10.1182/blood-2007-04-067215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brunstein CG, Gutman JA, Weisdorf DJ, Woolfrey AE, Defor TE, Gooley TA, et al. Allogeneic hematopoietic cell transplantation for hematologic malignancy: relative risks and benefits of double umbilical cord blood. Blood. 2010;116(22):4693–4699. doi: 10.1182/blood-2010-05-285304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brunstein CG, Laughlin MJ. Extending cord blood transplant to adults: dealing with problems and results overall. Seminars in hematology. 2010;47(1):86–96. doi: 10.1053/j.seminhematol.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 6.Scaradavou A, Brunstein CG, Eapen M, Le-Rademacher J, Barker JN, Chao N, et al. Double unit grafts successfully extend the application of umbilical cord blood transplantation in adults with acute leukemia. Blood. 2013;121(5):752–758. doi: 10.1182/blood-2012-08-449108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.MacMillan ML, Weisdorf DJ, Brunstein CG, Cao Q, DeFor TE, Verneris MR, et al. Acute graft-versus-host disease after unrelated donor umbilical cord blood transplantation: analysis of risk factors. Blood. 2009;113(11):2410–2415. doi: 10.1182/blood-2008-07-163238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maris MB, Niederwieser D, Sandmaier BM, Storer B, Stuart M, Maloney D, et al. HLA-matched unrelated donor hematopoietic cell transplantation after nonmyeloablative conditioning for patients with hematologic malignancies. Blood. 2003;102(6):2021–2030. doi: 10.1182/blood-2003-02-0482. [DOI] [PubMed] [Google Scholar]
- 9.Giaccone L, McCune JS, Maris MB, Gooley TA, Sandmaier BM, Slattery JT, et al. Pharmacodynamics of mycophenolate mofetil after nonmyeloablative conditioning and unrelated donor hematopoietic cell transplantation. Blood. 2005;106(13):4381–4388. doi: 10.1182/blood-2005-06-2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maris MB, Sandmaier BM, Storer BE, Maloney DG, Shizuru JA, Agura E, et al. Unrelated donor granulocyte colony-stimulating factor-mobilized peripheral blood mononuclear cell transplantation after nonmyeloablative conditioning: the effect of postgrafting mycophenolate mofetil dosing. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2006;12(4):454–465. doi: 10.1016/j.bbmt.2005.12.030. [DOI] [PubMed] [Google Scholar]
- 11.Warlick ED, Tomblyn M, Cao Q, Defor T, Blazar BR, Macmillan M, et al. Reduced-intensity conditioning followed by related allografts in hematologic malignancies: long-term outcomes most successful in indolent and aggressive non-Hodgkin lymphomas. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2011;17(7):1025–1032. doi: 10.1016/j.bbmt.2010.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McDermott CL, Sandmaier BM, Storer B, Li H, Mager DE, Boeckh MJ, et al. Nonrelapse mortality and mycophenolic acid exposure in nonmyeloablative hematopoietic cell transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2013;19(8):1159–1166. doi: 10.1016/j.bbmt.2013.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jacobson P, Rogosheske J, Barker JN, Green K, Ng J, Weisdorf D, et al. Relationship of mycophenolic acid exposure to clinical outcome after hematopoietic cell transplantation. Clinical pharmacology and therapeutics. 2005;78(5):486–500. doi: 10.1016/j.clpt.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 14.Jacobson P, El-Massah SF, Rogosheske J, Kerr A, Long-Boyle J, DeFor T, et al. Comparison of two mycophenolate mofetil dosing regimens after hematopoietic cell transplantation. Bone marrow transplantation. 2009;44(2):113–120. doi: 10.1038/bmt.2008.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rogosheske JR, Fargen AD, DeFor TE, Warlick E, Arora M, Blazar BR, et al. Higher therapeutic CsA levels early post transplantation reduce risk of acute GVHD and improves survival. Bone marrow transplantation. 2014;49(1):122–125. doi: 10.1038/bmt.2013.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2005;11(12):945–956. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 17.Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone marrow transplantation. 1995;15(6):825–828. [PubMed] [Google Scholar]
- 18.van Burik JA, Carter SL, Freifeld AG, High KP, Godder KT, Papanicolaou GA, et al. Higher risk of cytomegalovirus and aspergillus infections in recipients of T cell-depleted unrelated bone marrow: analysis of infectious complications in patients treated with T cell depletion versus immunosuppressive therapy to prevent graft-versus-host disease. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2007;13(12):1487–1498. doi: 10.1016/j.bbmt.2007.08.049. [DOI] [PubMed] [Google Scholar]
- 19.Sorror ML, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106(8):2912–2919. doi: 10.1182/blood-2005-05-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaplan ELMP. Nonparametric estimation from incomplete observations. Journal of the American Statistical Association. 1958;53:457–481. [Google Scholar]
- 21.Cox DR. Regression models and life tables. Journal of the Royal Stastistical Society. 1972:187–220. [Google Scholar]
- 22.Lin DY. Non-parametric inference for cumulative incidence functions in competing risks studies. Statistics in medicine. 1997;16(8):901–910. doi: 10.1002/(sici)1097-0258(19970430)16:8<901::aid-sim543>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 23.Fine JPG, R J. A proportional hazards model for the subdistribution of a competing risk. Journal of the American Statistical Association. 1999;94:496–509. [Google Scholar]
- 24.Zhang X, Zhang MJ. SAS macros for estimation of direct adjusted cumulative incidence curves under proportional subdistribution hazards models. Comput Methods Programs Biomed. 2011;101:87–93. doi: 10.1016/j.cmpb.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18(6):695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 26.Harnicar SJ, Ponce DM, Gregornik D, Mathew S, Evans KL, Zheng JT, et al. Higher Mycophenolic Acid (MPA) Trough Levels Result In Lower Day 100 Severe Acute GVHD Without Increased Toxicity In Double-Unit Cord Blood Transplantation (CBT) Recipients. Blood. 2013;122(21) [Google Scholar]
- 27.Brunstein CG, Fuchs EJ, Carter SL, Karanes C, Costa LJ, Wu J, et al. Alternative donor transplantation after reduced intensity conditioning: results of parallel phase 2 trials using partially HLA-mismatched related bone marrow or unrelated double umbilical cord blood grafts. Blood. 2011;118(2):282–288. doi: 10.1182/blood-2011-03-344853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ciurea SO, Mulanovich V, Saliba RM, Bayraktar UD, Jiang Y, Bassett R, et al. Improved early outcomes using a T cell replete graft compared with T cell depleted haploidentical hematopoietic stem cell transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2012;18(12):1835–1844. doi: 10.1016/j.bbmt.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li H, Mager DE, Sandmaier BM, Maloney DG, Bemer MJ, McCune JS. Population pharmacokinetics and dose optimization of mycophenolic acid in HCT recipients receiving oral mycophenolate mofetil. Journal of clinical pharmacology. 2013;53(4):393–402. doi: 10.1002/jcph.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eapen M, Klein JP, Sanz GF, Spellman S, Ruggeri A, Anasetti C, et al. Effect of donor-recipient HLA matching at HLA A, B, C, and DRB1 on outcomes after umbilical-cord blood transplantation for leukaemia and myelodysplastic syndrome: a retrospective analysis. The lancet oncology. 2011;12(13):1214–1221. doi: 10.1016/S1470-2045(11)70260-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ponce DM, Gonzales A, Lubin M, Castro-Malaspina H, Giralt S, Goldberg JD, et al. Graft-versus-host disease after double-unit cord blood transplantation has unique features and an association with engrafting unit-to-recipient HLA match. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2013;19(6):904–911. doi: 10.1016/j.bbmt.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brunstein CGD, T E, Noreen H, Maurer D, MacMillan ML, Verneris MR, McGlave P, Weisdorf D, Wagner JE. Allele Level HLA Matching On Outcomes After Double Umbilical Cord Blood (dUCB) Transplantation for Hematological Malignancies: Stronger Graft Vs. Leukemia with HLA Mismatch. Blood (ASH Annual Meeting Abstracts) 2012;120(21):1976. [Google Scholar]