Figure 2.
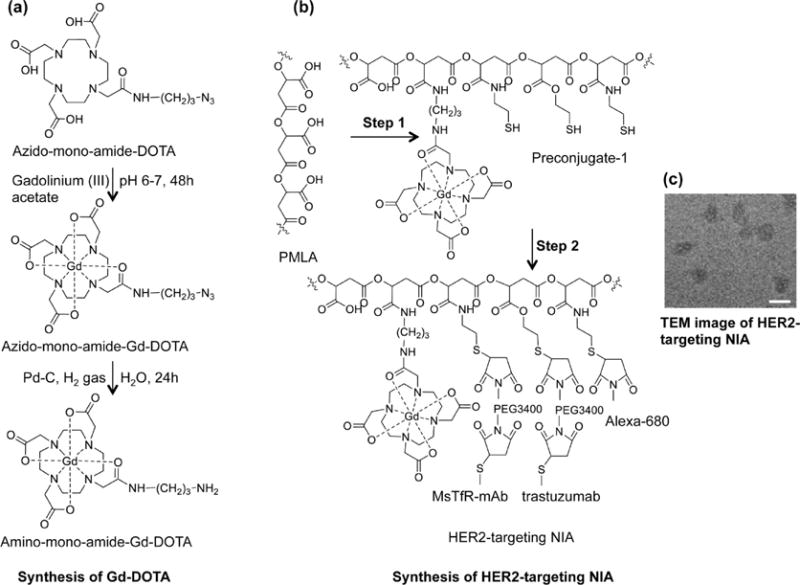
Synthesis of nanoimaging agent for MRI and fluorescent imaging (HER-2 targeting NIA is shown). (a) Synthesis of Gd-DOTA amine. Metal complex was prepared by reacting azido-monoamide-DOTA with 1.1 molar excess of gadolinium(III) acetate while maintaining the pH between 6 and 7. Azido-monoamide-Gd-DOTA was dissolved in water (25 mg/mL), and hydrogenolysis was carried out by hydrogen gas in the presence of 10% (w/w) Pd–C (palladium on carbon). The product (amino-monoamide-Gd-DOTA) was used for further reactions without any additional purification. (b) Step 1: Attachment of Gd-DOTA and 2-mercapto-1-ethylamine (MEA) through amide linkage after N-hydroxysuccinimide (NHS) activation of PMLA’s pendant carboxylates. The product (Preconjugate-1) was purified by size exclusion chromatography and obtained as a white floppy solid after lyophilization. Preconjugate-1 is stable at −20 °C for months without measurable loss in chemical or physicochemical reactivity. Step 2: Maleimide-functionalized mAbs and Alexa-680 were conjugated by a stable thioether bond. Trastuzumab was chosen for HER2 targeting (as shown), and cetuximab was chosen for EGFR targeting (not shown here). Anti-mouse TfR (MsTfR) mAb is used for tumor endothelial binding and transcytosis. Finally excess thiol groups masked using PDP and products were purified by PD-10 columns. (c) TEM image of HER2-targeting NIA. Scale bar = 25 nm.
