Scheme 1. Synthesis of Substituted 2-(Isoxazol-3-yl)-2-oxo-N′-phenyl-acetohydrazonoyl Cyanide Analogues with Modification on the Phenyl and Isoxazol Ringsa.
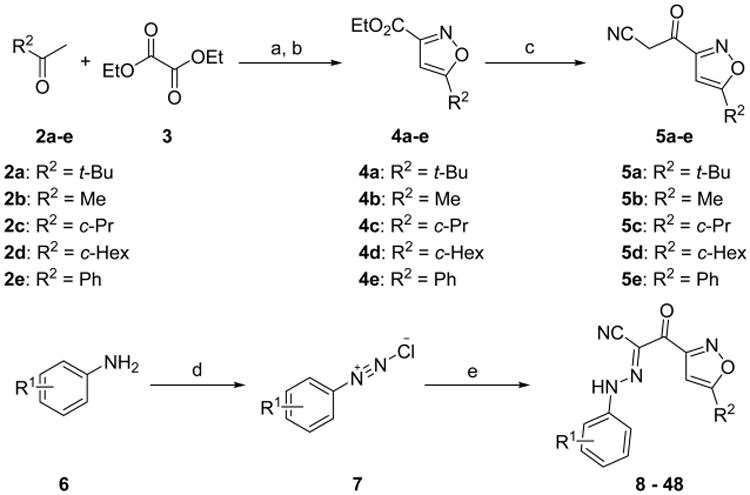
aReagents and conditions: (a) NaH, THF, 0 °C to rt; (b) NH2OH·HCl, EtOH/THF, rt to reflux, 30–87% for two steps; (c) CH3CN, MeLi, THF, −78 °C; (d) 2 N HCl, NaNO2, H2O, 0 °C; (e) 5a–e, NaOAc, EtOH, 24–76% for three steps.
