Table 1. Apparent IC50 Values of Substituted 2-(Isoxazol-3-yl)-2-oxo-N′-phenyl-acetohydrazonoyl Cyanide Scaffolds with Modifications on the Phenyl Ring for Competing with 8-NBD-cAMP in Binding to EPAC2.
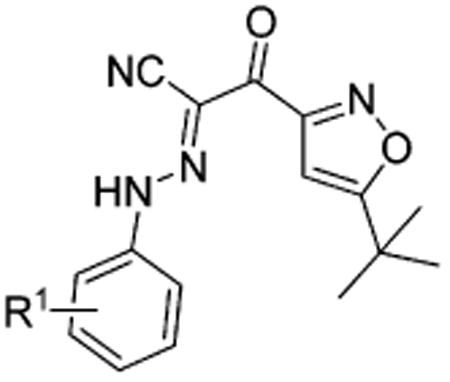
| |||
|---|---|---|---|
|
| |||
| Compd | R1 | IC50 (μM)a | relative affinity (RA)b |
| cAMP | 32.0 ± 5.9 | 1.0 | |
| 1 | 3-Cl | 8.9 ± 1.2 | 3.6 |
| 8 | H | 59.8 ± 9.4 | 0.5 |
| 9 | 2-Cl | 28.3 ± 4.0 | 1.1 |
| 10 | 4-Cl | 26.5 ± 4.1 | 1.2 |
| 11 | 3-Br | 14.0 ± 2.4 | 2.3 |
| 12 | 3-F | 22.4 ± 2.8 | 1.4 |
| 13 | 3-CF3 | 11.9 ± 1.7 | 2.7 |
| 14 | 3-NO2 | 31.3 ± 5.5 | 1.0 |
| 15 | 3-COOEt | 71.4 ± 23.8 | 0.4 |
| 16 | 3-CN | 67.8 ± 23.5 | 0.5 |
| 17 | 3-COCH3 | NEc | |
| 18 | 3-OMe | NE | |
| 19 | 3-CH2OH | NE | |
| 20 | 3-Me | 25.1 ± 4.8 | 1.3 |
| 21 | 2,5-di-Cl | 7.3 ± 1.2 | 4.4 |
| 22 | 3,5-di-Cl | 1.9 ± 0.339 | 16.8 |
| 23 | 3,4-di-Cl | 3.0 ± 0.6 | 10.7 |
| 24 | 2,3-di-Cl | 35.6 ± 7.9 | 0.9 |
| 25 | 3,5-di-CF3 | 6.4 ± 0.9 | 5.0 |
| 26 | 3-F, 5-CF3 | 5.8 ± 0.9 | 5.5 |
| 27 | 3,5-di-F | 5.3 ± 0.8 | 6.0 |
| 28 | 3-Cl, 5-CF3 | 1.6 ± 0.3 | 20.0 |
| 29 | 3-F, 5-Cl | 2.5 ± 0.4 | 12.8 |
| 30 | 3-F, 4-Cl | 4.3 ± 0.5 | 7.4 |
| 31 | 3-Cl, 4-F | 2.7 ± 0.3 | 11.9 |
| 32 | 3-CF3, 4-Cl | 2.9 ± 0.5 | 11.0 |
| 33 | 3,4-di-F | 6.6 ± 0.8 | 4.8 |
| 34 | 3-Cl, 4-CF3 | 3.6 ± 0.6 | 8.9 |
| 35 | 3,4,5-tri-Cl | 0.9 ± 0.2 | 35.6 |
| 36 | 3,4,5-tri-F | 1.1 ± 0.2 | 29.1 |
| 37 | 2,4,6-tri-Cl | 19.0 ± 1.7 | 1.7 |
The values are the mean ± SE of at least three independent experiments.
RA = IC50,cAMP/IC50,compound.
NE: no effect, indicating that a specific IC50 cannot be calculated from the data points collected.
