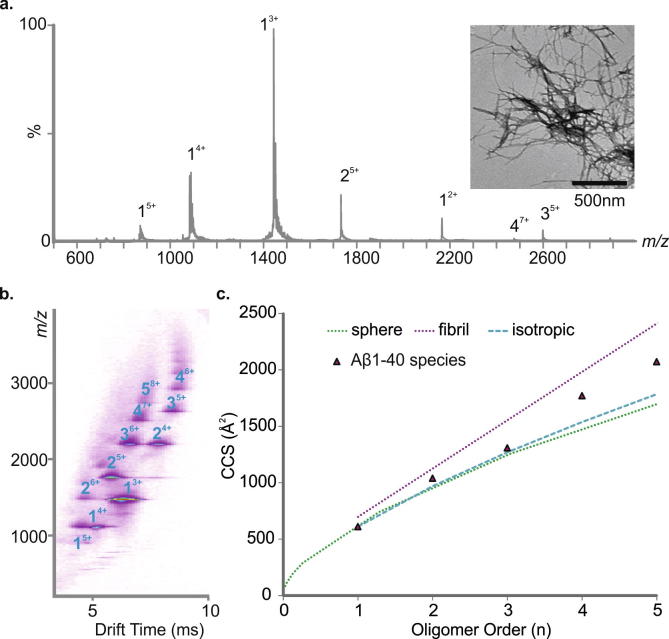
Fig. 2.
Analysis of Aβ40 oligomer distribution and collision-cross section (CCS). (a) ESI–MS mass spectrum of Aβ40. Numbers above peaks denote oligomer order, with the positive charge state of ions in superscript. Inset: negative stain TEM image of Aβ40 fibrils after 5 days in 200 mM ammonium acetate buffer, pH 6.8 (25 °C, quiescent) (scale bar = 500 nm). (b) ESI-IMS–MS Driftscope plot of the Aβ40 oligomers present 2 min after diluting the monomer to a final peptide concentration of 32 μM in 200 mM ammonium acetate, pH 6.8, 25 °C. ESI-IMS–MS Driftscope plots show IMS drift time versus m/z versus intensity (z = square root scale); (c) CCSs of Aβ40 oligomers measured using ESI-IMS–MS versus oligomer order; the CCS of the lowest charge state of each oligomer is shown (black triangles). The green dashed line represents a fit based on globular oligomers and the average density of a protein (0.44 Da/Å3) [28], the purple dashed line represents a linear growth model [8] and the blue dashed line represents an isotropic growth model [8].