Summary
BACKGROUND
The safety and efficacy of frontline nilotinib and dasatinib in newly diagnosed chronic-phase chronic myelogenous leukemia (CML-CP) patients with pre-existing liver and/or renal dysfunction are unknown.
PATIENTS and METHODS
We analyzed adverse event rates, response rates, and survival rates of 215 CML-CP patients with or without renal and/or liver dysfunction who were treated with front-line nilotinib (108 patients) or dasatinib (107 patients).
RESULTS
Overall median follow-up was 49 months. At baseline, 6 (6%) dasatinib-treated patients had mild renal dysfunction and 13 (12%) had mild liver dysfunction. Eight (7%) nilotinib-treated patients had mild renal dysfunction, 1 (1%) had moderate renal dysfunction, and 9 (8%) mild liver dysfunction. There were no significant differences in the rates of complete cytogenetic response, major molecular response, or MR4.5 between organ function cohorts. Dasatinib- or nilotinib- treated patients with baseline renal dysfunction had a higher incidence of transient reversible acute kidney injury (p=0.011; p<0.001), and nilotinib-treated patients with renal dysfunction had a higher incidence of bleeding (p<0.001).
CONCLUSION
CML-CP patients with mild to moderate renal or liver dysfunction can be safely treated with frontline dasatinib or nilotinib and can achieve response rates similar to those of CML-CP patients with normal organ function.
Keywords: nilotinib, dasatinib, chronic myelogenous leukemia, liver dysfunction, renal dysfunction
Introduction
Nilotinib and dasatinib—orally administered selective tyrosine kinase inhibitors that target BCR-ABL kinase and several other kinases1–3—are standard frontline treatments for patients with chronic myeloid leukemia (CML).4–14 In the phase 3, randomized, open-label, multicenter ENESTnd trial (Evaluating Nilotinib Efficacy and Safety in Clinical Trials-Newly Diagnosed Patients) and the multinational randomized DASISION trial (DASatinib versus Imatinib Study In treatment-Naïve CML patients), the efficacy and safety of nilotinib and dasatinib was compared with that of imatinib in patients with newly diagnosed CML in chronic phase (CML-CP).6 Analysis of long-term follow-up data confirmed that, compared with imatinib, nilotinib and dasatinib induced a higher rate of molecular responses, responses were deeper and were achieved faster, and were associated with fewer instances of progression to accelerated or blast phases in patients with newly diagnosed CML-CP.7, 8, 9, 10
Nilotinib and dasatinib are well absorbed orally with 31% and 19% bioavailability, respectively and are mainly metabolized by liver through oxidation and hydroxylation via CYP3A4 to primarily inactive metabolites excreted to bile duct.15, 16 Although both drugs are generally well tolerated, both can cause various hematologic and nonhematologic adverse events. In the ENESTnd trial, common nonhematologic adverse events observed wit nilotinib included rash, headache, nausea, alopecia, pruritus, myalgia, fatigue, and vomiting. Common biochemical abnormalities included increased levels of total bilirubin, aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), amylase, lipase, and creatinine.6 In the DASISION trial, fluid retention (including pleural effusion), myalgia, nausea, diarrhea, vomiting, rash, headache, and fatigue were observed with dasatinib.10 Grade 3 or 4 biochemical abnormalities included elevated levels of AST, ALT, total bilirubin, and creatinine.9
ENESTnd and DASISION both had strict inclusion criteria including adequate renal and liver function. However, frequently patients present at the time of diagnosis of CML with mild to moderate liver or renal dysfunction. There is no currently available data on the safety and efficacy of nilotinib and dasatinib in this patient population. The purpose of the present analysis is to determine the safety and efficacy of nilotinib and dasatinib in CML-CP patients with pre-existing liver and/or renal dysfunction.
Methods
Patients
For this analysis we included 215 consecutive previously untreated CML-CP patients enrolled in concomitant phase 2 clinical trials of dasatinib or nilotinib between May 2005 and October 2012 at MD Anderson Cancer Center. 4, 10 These trials were registered at www.clinicaltrials.gov as NCT00254423 and NCT00129740. The starting doses of nilotinib and dasatinib were 400 mg twice daily, and 100 mg daily or 50 mg twice daily, respectively. Inclusion criteria of the clinical trials included Philadelphia chromosome positive or BCR-ABL positive CML-CP diagnosed within 12 months before enrollment. CP was defined as <15% blasts, <20% basophils, and <30% blasts and promyelocytes in the blood or bone marrow; no extramedullary disease; and platelet count > 100 × 109 /L (unrelated to therapy). Patients were also required to be at least 15 years old, have an Eastern Cooperative Oncology Group performance status of 0–2, and have a total bilirubin ≤1.5 x the upper limit of normal (ULN), an ALT ≤2.5 x ULN, and creatinine ≤1.5 x ULN. All patients signed an informed consent that had been approved by the Institutional Review Board in accordance with the Declaration of Helsinki.
Classification of Renal and Liver Function
Renal function and liver function were classified according to guidelines from the National Cancer Institute Organ Dysfunction Working Group. Creatinine clearance (CrCl) was estimated with the Modification of Diet in Renal Disease (MDRD) method from the national kidney disease education program [estimated glomerular filtration rate (mL/min/1.73 m2) = 175 × (Scr)−1.154 × (Age)−0.203 × (0.742 if female) × (1.212 if African American)].17 Normal renal function was defined as a CrCl of ≥60 mL/min; mild renal dysfunction as a CrCl of 40–59 mL/min; moderate renal dysfunction as a CrCl of 20–39 mL/min; and severe renal dysfunction as a CrCl of ≤20 mL/min. Normal liver function was defined as a total bilirubin level ≤ULN and an AST level the ≤ULN; mild liver dysfunction as a total bilirubin level ≤1.5 x the ULN and an AST level > ULN; moderate liver dysfunction as a total bilirubin level 1.5 to 3.0 x the ULN and an AST level of any value; and severe liver dysfunction as a total bilirubin level > 3.0 x the ULN and an AST of any value.
Monitoring and Assessment of Response
Hematologic, cytogenetic, and molecular response criteria were defined as described previously.18, 19 Briefly, a complete hematologic remission was defined as normalization of the bone marrow (less than 5% blasts) and peripheral blood (white blood cell count <10 × 109/L and no peripheral blasts, promyelocytes, or myelocytes) for at least 4 weeks. Complete cytogenetic responses were based on the absence of Philadelphia chromosome–positive metaphases with at least 20 metaphases analyzed. Major molecular response (MMR) and MR4.5 was defined as a BCR-ABL/ABL ratio of ≤0.1% and ≤0.0032% on the international scale (IS), respectively. Molecular response 4.5 (MR4.5) was defined as BCR-ABL/ABL ratio ≤0.0032% IS.
Survival
Survival duration was measured from the start of nilotinib or dasatinib until death from any cause, and patients who were alive at the end of the study period were censored at the date of last follow-up. Event-free survival (EFS) was calculated from the start of nilotinib or dasatinib to loss of complete hematologic response, loss of major cytogenetic response, transformation to accelerated or blast phase, or death from any cause. Failure free survival (FFS) was calculated from the start of nilotinib or dasatinib to discontinuation or switch to another treatment for any reason. Treatment failure survival (TFS) was calculated from the start of therapy to transformation to AP or BP, or death. For EFS and PFS, patients who discontinued therapy for other reasons (e.g., noncompliance, financial issues) or who were lost to follow-up were censored at the date of last treatment, and patients who were still receiving either agent at the end of the study period were censored at the date of last follow-up. Telephone surveys were conducted to determine whenever possible the reasons patients were lost to follow-up.
Statistical Analysis
The primary objective of the present study was to analyze the toxicity profiles and response rates of nilotinib- or dasatinib-treated CML-CP patients with normal organ function or liver and/or renal dysfunction. Secondary endpoints included 4-year FFS, TFS, EFS and OS rates. The Fisher exact test and Mann-Whitney U test were used to assess differences between groups. The main analysis for the primary and secondary endpoints was performed in the intention-to-treat population. Four-year FFS, TFS, EFS and OS rates were estimated using the Kaplan-Meier method and analyzed using the log-rank test. All reported P values were 2-sided, and P values <0.05 were considered statistically significant. Cox proportional hazards regression for survival was used for univariate (UVA) and multivariate analysis (MVA). Statistical significant variables with UVA were proceeded to MVA. All statistical analyses were performed using the SPSS version 22 software program.
Results
Patients
The median follow-up duration was 49 months (range, 3–93 months). The clinical characteristics of all patients are summarized in Table 1. A total of 15 (7%) patients had renal dysfunction (14 mild, one moderate) and 22 (10%) liver dysfunction. Per the study’s eligibility criteria, patients with severe liver dysfunction were excluded from the studies. One patient in each cohort had both mild renal and mild liver dysfunction. According to treatment received, 6 (6%) of the 107 dasatinib-treated patients had mild renal dysfunction and 13 (12%) had mild liver dysfunction. Among the 108 patients treated with nilotinib, 8 (7%) had mild renal dysfunction, 1 (1%) moderate renal dysfunction, and 9 (8%) mild liver dysfunction. The median age at diagnosis was significantly higher in the renal dysfunction cohort (59 years) compared to those with normal liver and renal function or with liver dysfunction (48 and 46 years, respectively; p<0.001). Of the 15 patients with pre-existing renal dysfunction, 10 (67%) had low and 5 (33%) intermediate Sokal risk scores. Of the 22 patients with pre-existing liver dysfunction, 18 (82%) had low, 3 (14%) intermediate, and 1 (5%) high Sokal risk scores.20, 21
Table 1.
Patient characteristics by baseline organ function
| Characteristic | Normal N=180 |
Liver* N=22 |
Renal* N=15 |
Overall N=215 |
P |
|---|---|---|---|---|---|
| Median age, y (range) | 48 (16–83) | 46 (20–65) | 59 (50–86) | 48 (16–86) | <0.001 |
| Male sex, No. (%) | 103 (57) | 14 (64) | 12 (80) | 128 (60) | 0.206 |
| Median follow-up, month (range) | 48 (3–92) | 60 (17–88) | 53 (12–93) | 49 (3–93) | 0.071 |
| Sokal risk score, No. (%) | |||||
| Low | 134 (74) | 18 (82) | 10 (67) | 160 (74) | 0.545 |
| Intermediate | 35 (19) | 3 (14) | 5 (33) | 43 (20) | |
| High | 11 (6) | 1 (5) | 0 | 12 (6) | |
| Organ dysfunction, No. (%) | |||||
| Normal | 180 (100) | 0 | 0 | 178 (83) | <0.001 |
| Mild | 0 | 22 (100) | 14 (93) | 36 (17) | |
| Moderate | 0 | 0 | 1 (7) | 1 (0) | |
| Severe | 0 | 0 | 0 | 0 | |
| Type of TKI, No. (%) | |||||
| Nilotinib | 91 (51) | 9 (41) | 9 (60) | 108 (50) | 0.510 |
| Dasatinib | 89 (49) | 13 (59) | 6 (40) | 107 (50) | |
| CE at diagnosis, No. (%) | 23 (13) | 1 (5) | 3 (20) | 27 (13) | 0.962 |
| Time from diagnosis to treatment, day (range) | 21 (1–233) | 13 (1–86) | 14 (2–80) | 19 (1–233) | 0.685 |
Two patients had both mild liver and mild renal dysfunction at diagnosis.
Abbreviations: TKI, tyrosine kinase inhibitor; CE, clonal evolution, NA, not applicable.
Changes in Kidney Function after Dasatinib or Nilotinib Treatment
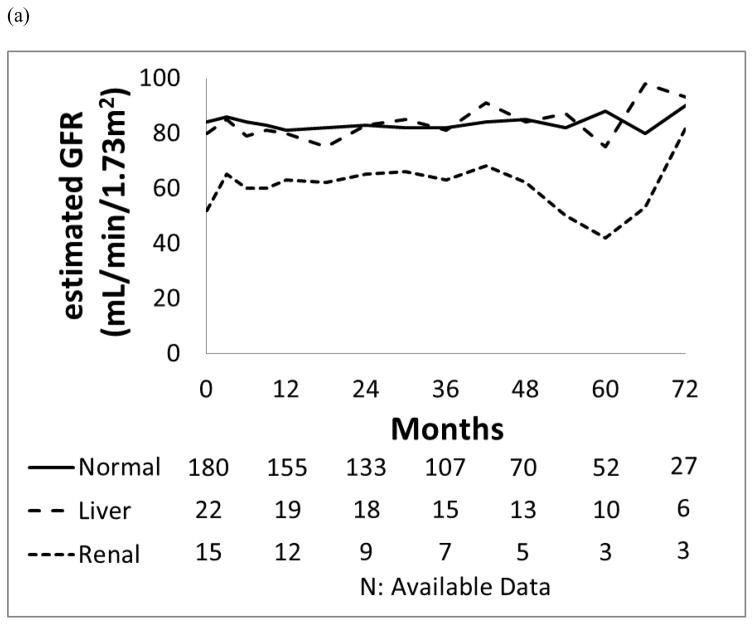
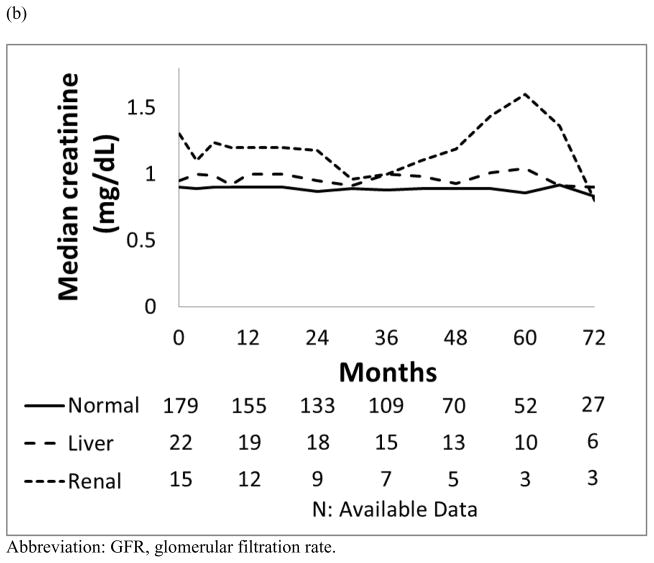
As a group, patients with pre-existing renal dysfunction generally maintained stable their renal function (Figure 1). Patients with baseline renal dysfunction who were treated with dasatinib or nilotinib had a higher incidence of all grade acute kidney injury, occurring in 3 (50%) and 6 (67%) patients, respectively, compared to those with normal organ function (8 −9% and 11 −12%; p=0.011; p<0.001, respectively).
Figure 1.
Median values for the trend of renal function: (a) estimated GFR (mL/min/1.73m2) and (b) Creatinine (mg/dL) in each organ function group.
Of the 107 patients treated with dasatinib, 13 (12%) developed all grades renal toxicities as follows: 8 (9%) had normal baseline organ function, 2 (15%) had baseline liver dysfunction and 3 (50%) had baseline renal dysfunction. Of the 6 patients with pre-existing renal dysfunction in dasatinib cohort, 3 (50%) had transient decreases in their estimated GFR from a median of 53 mL/min (range, 49–59 mL/min) at baseline while maintaining mild renal dysfunction. Worsening renal function among these 13 patients was usually transient with 12 patients recovering with oral hydration; 1 patient required temporary discontinuation of dasatinib for 5 days with complete resolution of kidney function, and later tolerated recommencement of dasatinib therapy at the same dose.
Of the 108 patients treated with nilotinib, 18 (17%) experienced all grades kidney toxicities as follows: 11 (12%) had normal baseline organ function, 1 (11%) had baseline liver dysfunction and 6 (67%) had baseline renal dysfunction. Of 18 patients who had renal toxicities, 16 patients recovered with oral hydration without nilotinib interruption, and 2 patients required transient discontinuation of nilotinib for 5 days and 7 days, respectively, which resulted in complete recovery of renal function; these 2 patients tolerated the same dose of nilotinib upon resumption of therapy. Of the 9 patients who had mild or moderate renal dysfunction at the start of nilotinib treatment, 6 (67%) developed transient or persistent worsening renal dysfunction. This was reflected by a decrease in these patients’ median estimated GFR from of 52 mL/min (range, 30–57 mL/min) at baseline. Eight patients (89%) maintained mild renal dysfunction, and 1 patient (11%) progressed to moderate renal dysfunction.
Changes in Liver Function after Dasatinib or Nilotinib Treatment
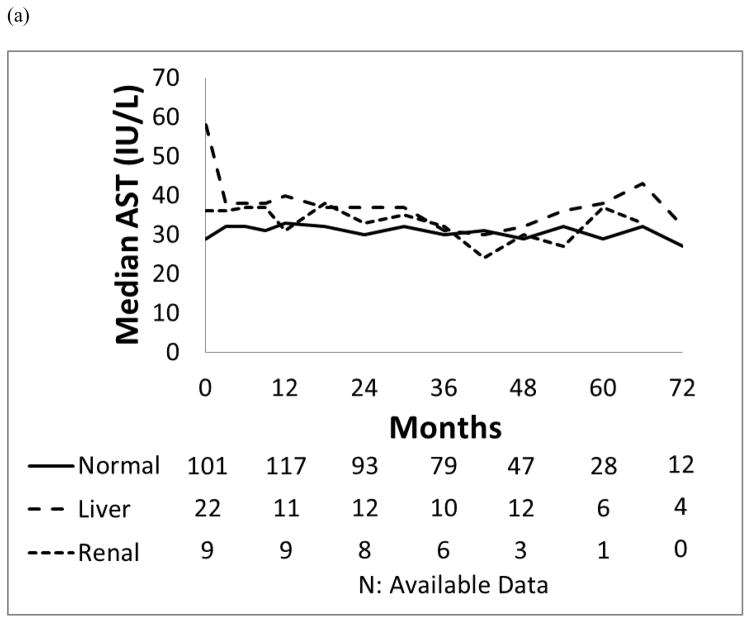
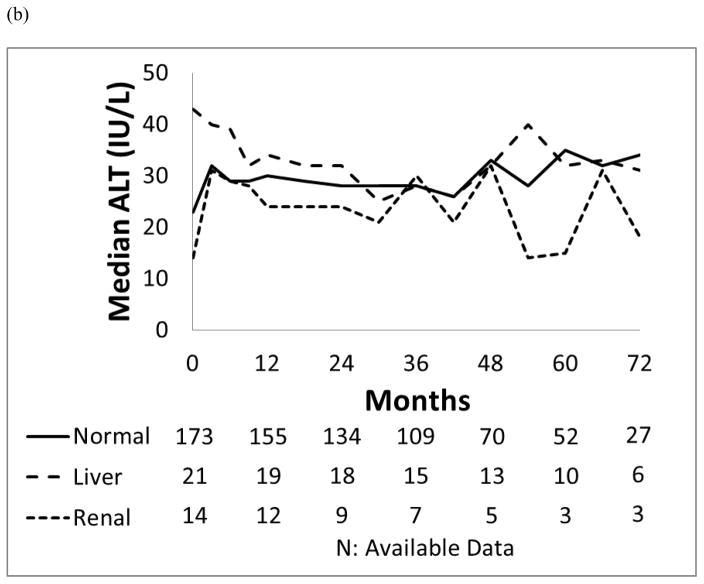
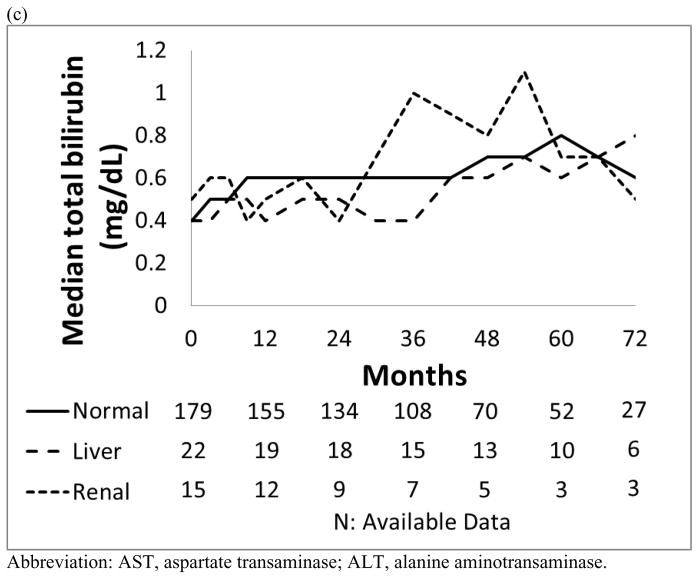
Patients with liver dysfunction who were treated with dasatinib or nilotinib did not have a significantly higher incidence of all grade liver toxicity compared to those with normal organ function or renal dysfunction (p=0.459; p=0.723). Their bilirubin, ALT and AST levels remained generally unchanged throughout the study period (Figure 2).
Figure 2. Median values for the trend of liver function.
(a) AST (IU/L), (b) ALT (IU/L) and (c) Total bilirubin (mg/dL) in each organ function group.
In the dasatinib cohort, 58 patients (54%) developed all grade liver toxicities including 45 (51%) who had normal baseline organ function, 10 (77%) who had baseline liver dysfunction and 4 (67%) who had baseline renal dysfunction. One patient who developed liver toxicity had both pre-existing liver and renal dysfunction. Of the 13 patients with mild liver dysfunction at the start of dasatinib treatment, 10 (77%) experienced transient grade 1 liver toxicities, 3 (23%) had total bilirubin elevation, and 9 (69%) and AST/ALT/ALP elevation. Liver toxicities in these 58 patients resolved without medical intervention, interruption, or dose reduction of dasatinib.
In the nilotinib cohort, 87 (81%) developed liver toxicities: 72 (79%) who had normal baseline organ function, 8 (89%) with baseline liver dysfunction and 7 (78%) with baseline renal dysfunction. Grade 3/4 hyperbilirubinemia was observed in 8 (9%) in the group with normal baseline organ function, and was not observed among patients with baseline renal or liver dysfunction. Of 9 patients with pre-existing liver impairment, 8 (89%) had grade 1/2 liver toxicities without episodes of grade 3/4 liver toxicities. Among the 12 patients who developed grade 3/4 liver toxicities, 8 required nilotinib interruption with median duration of 18 days (range, 3–64 days), and required dose reduction of nilotinib. Four of these 12 patients with grade 3/4 liver toxicities had isolated indirect-predominant hyperbilirubinemia.
Other Toxicity Profile
We then analyzed the overall toxicity profiles of patients with or without pre-existing organ dysfunction who were treated with dasatinib (Table 2) or nilotinib (Table 3). Treatment was generally well tolerated. Overall, 44 patients (24%) in the normal organ function cohort and 12 (33%) in the organ dysfunction cohort discontinued therapy, including 1 patient with both renal and liver dysfunction.
Table 2.
Treatment-emergent adverse events with dasatinib by organ function
| Toxicity, No. (%) | Normal N = 89 |
Liver dysfunction* N = 13 |
Renal dysfunction* N = 6 |
P | |||
|---|---|---|---|---|---|---|---|
| All | G 3/4 | All | G 3/4 | All | G 3/4 | ||
| Anemia | 80 (90) | 5 (6) | 13 (100) | 1 (8) | 6 (100) | 0 | 0.629 |
| Neutropenia | 39 (44) | 15 (17) | 7 (54) | 2 (15) | 1 (17) | 0 | 0.583 |
| Thrombocytopenia | 51 (57) | 6 (7) | 6 (46) | 2 (15) | 4 (67) | 0 | 0.494 |
| Lipase | 5 (6) | 1 (1) | 0 | 0 | 0 | 0 | 0.901 |
| Rash | 47 (53) | 0 | 7 (54) | 0 | 3 (50) | 0 | 0.988 |
| Edema | 32 (36) | 1 (1) | 2 (15) | 0 | 3 (50) | 0 | 0.558 |
| Nausea/vomiting | 33 (37) | 1 (1) | 4 (31) | 0 | 2 (33) | 0 | 0.984 |
| Diarrhea | 31 (35) | 0 | 6 (46) | 0 | 1 (17) | 0 | 0.451 |
| Liver toxicity | 45 (51) | 0 | 10 (77) | 0 | 4 (67) | 0 | 0.459 |
| Total bilirubin | 17 (19) | 0 | 3 (23) | 0 | 1 (17) | 0 | 0.930 |
| AST/ALT/ALP | 39 (44) | 0 | 9 (69) | 0 | 3 (50) | 0 | 0.546 |
| Muscle cramp | 34 (38) | 3 (3) | 6 (46) | 1 (8) | 2 (33) | 0 | 0.909 |
| Kidney toxicity | 8 (9) | 0 | 2 (15) | 0 | 3 (50) | 0 | 0.011 |
| Pleural effusion | 20 (23) | 8 (9) | 3(23) | 1 (8) | 3 (50) | 2 (33) | 0.408 |
| Bleeding | 22 (25) | 4 (4) | 3 (23) | 0 | 1 (17) | 0 | 0.911 |
| QTc prolongation | 16 (18) | 2 (2) | 1 (8) | 0 | 1 (17) | 0 | 0.897 |
One patient had both renal and liver dysfunction.
Abbreviations: G, grade; AST, aspartate transaminase, ALT, alanine transaminase, ALP, alkaline phosphatase.
Table 3.
Treatment-emergent adverse events with nilotinib by organ function
| Toxicity, No. (%) | Normal N = 91 |
Liver dysfunction* N = 9 |
Renal dysfunction* N = 9 |
P | |||
|---|---|---|---|---|---|---|---|
| All | G 3/4 | All | G 3/4 | All | G 3/4 | ||
| Anemia | 73 (80) | 7 (8) | 7 (78) | 0 | 9 (100) | 1 (11) | 0.560 |
| Neutropenia | 22 (24) | 10 (11) | 0 | 0 | 2 (22) | 2 (22) | 0.287 |
| Thrombocytopenia | 34 (37) | 5 (5) | 2 (22) | 1 (11) | 5 (56) | 2 (22) | 0.256 |
| Lipase | 13 (14) | 7 (8) | 1 (11) | 0 | 2 (22) | 0 | 0.409 |
| Rash | 53 (58) | 2 (2) | 5 (56) | 0 | 4 (44) | 0 | 0.916 |
| Edema | 6 (7) | 0 | 1 (11) | 0 | 2 (22) | 0 | 0.253 |
| Nausea/vomiting | 28 (31) | 0 | 4 (44) | 0 | 4 (44) | 0 | 0.530 |
| Diarrhea | 12 (13) | 0 | 0 | 0 | 3 (33) | 0 | 0.113 |
| Liver toxicity | 72 (79) | 11 (12) | 8 (89) | 0 | 7 (78) | 1 (11) | 0.723 |
| Total bilirubin | 62 (68) | 8 (9) | 6 (67) | 0 | 6 (67) | 0 | 0.786 |
| AST/ALT/ALP | 60 (66) | 4 (4) | 8 (89) | 0 | 7 (78) | 1 (11) | 0.426 |
| Muscle cramp | 19 (21) | 0 | 2 (22) | 0 | 3 (33) | 0 | 0.691 |
| Kidney toxicity | 11 (12) | 0 | 1 (11) | 0 | 6 (67) | 1 (11) | <0.001 |
| Pleural effusion | 3 (3) | 2 (2) | 0 | 0 | 0 | 0 | 0.962 |
| Bleeding | 7 (8) | 0 | 2 (22) | 0 | 3 (33) | 2 (22) | <0.001 |
| QTc prolongation | 8 (10) | 0 | 1 (11) | 0 | 1 (11) | 0 | 0.953 |
One patient had both renal and liver dysfunction.
Abbreviations: G, grade; AST, aspartate transaminase; ALT, alanine transaminase; ALP, alkaline phosphatase.
Of 44 patients with normal organ function who discontinued therapy, 22 patients (50%) discontinued due to progression or resistance including 5 patients who required multiple dose reductions due to myalgia (1 patient), liver function test abnormalities (1 patient), thrombocytopenia (1 patient), and neutropenia (2 patients); 4 (9%) due to death including 1 from cardiovascular event while on dasatinib, 1 from a cardiovascular event while on nilotinib, 1 due to sepsis (on nilotinib), and 1 surgical complications after femur fracture; 4 (9%) discontinued for non-compliance; 3 (7%) due to insurance issues; 1 (2%) due to patient choice after diagnosis of pancreatic cancer; and 10 patients (23%) due to adverse events including 3 (on dasatinib) due to pleural effusion, 2 pancreatitis (both on nilotinib), and 1 each with congestive heart failure exacerbation (dasatinib), 1 myocardial infarction (on nilotinib), gastrointestinal hemorrhage (dasatinib), liver function test abnormalities (nilotinib), and pericarditis (nilotinib).
Of 7 patients with liver dysfunction, 3 patients (43%) discontinued front-line therapy due to resistance, 1 (14%) due to insurance issues, 1 (14%) for non-compliance and 2 (28%) for adverse events including 1 patient who had generalized weakness while on nilotinib, and 1 patient who had pleural effusion while on dasatinib. Of 6 patients with renal dysfunction, 1 (17%) discontinued due to resistance after multiple dose reduction related to liver function test abnormalities, 1 (17%) died from sepsis (with normal neutrophil count while on nilotinib), and 4 patients (67%) discontinued for adverse events including 2 with pleural effusion (both on dasatinib), 1 with generalized weakness (nilotinib), and 1 due to acute kidney injury (nilotinib). One patient with renal and liver dysfunction discontinued due to generalized weakness while on nilotinib.
The dosage of nilotinib and dasatinib at 12, 24, 36, 48, and 60 months is shown on Tables 4 and 5. Among patients with normal baseline organ function, 60 months after the start of therapy, 12 (44%) maintained their dose of dasatinib at 100 mg/day and 16 (55%) the planned daily dose of nilotinib of 800 mg. This compares to 0 (0%), and 3 (38%) for dasatinib (p=0.757); and 2 (100%), and 3 (75%) for nilotinib (p=0.181) for patients with renal and liver dysfunction, respectively.
Table 4.
Dose of dasatinib at specific time point.
| N | Dasatinib dose (mg/day) | P | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 140 | 100 | 80 | 70 | 60 | 50 | 40 | 20 | 10** | ||
| At 12 months, No. (%) | ||||||||||
| Normal N= 80 |
0 | 53 (66) | 7 (9) | 1 (1) | 0 | 4 (5) | 13 (16) | 2 (3) | 0 | 0.679 |
| Liver* N= 13 |
0 | 7 (54) | 4 (31) | 0 | 0 | 0 | 2 (15) | 0 | 0 | |
| Renal* N= 5 |
0 | 4 (80) | 0 | 0 | 0 | 0 | 1 (20) | 0 | 0 | |
| At 24 months, No. (%) | ||||||||||
| Normal N=67 |
1 (2) | 42 (63) | 8 (12) | 0 | 1 (2) | 0 | 12 (18) | 3 (5) | 0 | 0.613 |
| Liver* N=13 |
0 | 6 (46) | 5 (39) | 0 | 0 | 0 | 2 (15) | 0 | 0 | |
| Renal* N=5 |
0 | 2 (40) | 1 (20) | 0 | 0 | 0 | 2 (40) | 0 | 0 | |
| At 36 months, No. (%) | ||||||||||
| Normal N=56 |
1 (2) | 31 (55) | 7 (13) | 0 | 1 (2) | 2 (4) | 11 (20) | 2 (4) | 1 (2) | 0.584 |
| Liver* N=10 |
0 | 3 (30) | 2 (20) | 0 | 0 | 0 | 3 (30) | 2 (20) | 0 | |
| Renal* N=5 |
0 | 1 (20) | 0 | 0 | 0 | 0 | 3 (60) | 1 (20) | 0 | |
| At 48 months, No. (%) | ||||||||||
| Normal N=42 |
0 | 22 (52) | 6 (14) | 0 | 0 | 0 | 12 (29) | 2 (5) | 0 | 0.163 |
| Liver* N=8 |
0 | 2 (25) | 1 (13) | 0 | 0 | 0 | 3 (38) | 2 (25) | 0 | |
| Renal* N=3 |
0 | 0 | 0 | 0 | 0 | 0 | 2 (67) | 1 (33) | 0 | |
| At 60 months, No. (%) | ||||||||||
| Normal N=27 |
0 | 12 (44) | 6 (22) | 0 | 0 | 0 | 8 (30) | 1 (4) | 0 | 0.757 |
| Liver N=8 |
0 | 3 (38) | 1 (13) | 0 | 0 | 0 | 3 (38) | 1 (13) | 0 | |
| Renal N=1 |
0 | 0 | 0 | 0 | 0 | 0 | 1 (100) | 0 | 0 | |
One patient had both renal and liver dysfunction.
One patient had dasatinib at a dose of 20 mg every other day.
Table 5.
Dose of nilotinib at specific time point.
| Nilotinib dose (mg/day) | P | ||||
|---|---|---|---|---|---|
| 800 | 400 | 200 | 150 | ||
| At 12 months, No. (%) | |||||
| Normal N=78 |
53 (68) | 17 (22) | 7 (9) | 1 (1) | 0.703 |
| Liver N=7 |
6 (86) | 1 (14) | 0 | 0 | |
| Renal N=6 |
6 (100) | 0 | 0 | 0 | |
| At 24 months, No. (%) | |||||
| Normal N=71 |
42 (59) | 21 (30) | 8 (11) | 0 | 0.229 |
| Liver N=6 |
5 (83) | 0 | 1 (17) | 0 | |
| Renal N=5 |
5 (100) | 0 | 0 | 0 | |
| At 36 months, No. (%) | |||||
| Normal N=59 |
35 (59) | 16 (27) | 8 (14) | 0 | 0.289 |
| Liver N=5 |
4 (80) | 0 | 1 (20) | 0 | |
| Renal N=5 |
5 (100) | 0 | 0 | 0 | |
| At 48 months, No. (%) | |||||
| Normal N=39 |
23 (59) | 13 (33) | 3 (8) | 0 | 0.256 |
| Liver N=5 |
4 (80) | 0 | 1 (20) | 0 | |
| Renal N=4 |
4 (100) | 0 | 0 | 0 | |
| At 60 months, No. (%) | |||||
| Normal N=29 |
16 (55) | 12 (41) | 1 (3) | 0 | 0.181 |
| Liver N=4 |
3 (75) | 0 | 1 (25) | 0 | |
| Renal N=2 |
2 (100) | 0 | 0 | 0 | |
Two patients with renal dysfunction who received nilotinib experienced grade 3 or 4 bleeding in the setting of normal platelet counts; both had gastric ulcers (one of them associated with use of nonsteroidal anti-inflammatory agents) requiring blood transfusions.
Responses and Survival
Although patients with renal or liver dysfunction tended to have more treatment interruptions and dose reductions than patients with normal liver function, the BCR-ABL/ABL <10% on the international scale at 3 months, cumulative response within 1 year of TKI and best cumulative response rates of these groups did not differ significantly (Table 6). A CCyR was achieved in 167 (93%) patients with normal organ function and 20 (91%) and 13 (87%) with liver or renal dysfunction, respectively. Similarly, MMR was achieved in 154 (86%) patients with normal organ function and 19 (86%) and 12 (80%) with renal or liver dysfunction, and a MR4.5 was achieved in 124 (69%) patients with normal organ function and 9 (60%) and 14 (64%) with renal or liver dysfunction, respectively. Twelve months after the start of therapy, 166 patients (92%) with normal organ function, 20 (91%) with liver dysfunction, and 13 (87%) with renal dysfunction had a CCyR (normal vs. liver, p=0.830; normal vs. renal p=0.354).
Table 6.
Responses (expressed in percentage) to dasatinib or nilotinib by pre-existing organ function
| Response | Dasatinib* N=107 |
Nilotinib* N=108 |
Overall N=215 |
P | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Normal N=89 |
Liver N=13 |
Renal N=6 |
Normal N=91 |
Liver N=9 |
Renal N=9 |
Normal N=180 |
Liver N=22 |
Renal N=15 |
Normal vs. Liver | Normal vs. Renal | |
| BCR-ABL/ABL ratio on the international scale at 3 months | |||||||||||
| <10% | 78 | 77 | 100 | 87 | 89 | 89 | 82 | 82 | 93 | 0.963 | 0.474 |
| Cumulative response within 1 year, % | |||||||||||
| CCyR | 91 | 100 | 100 | 93 | 78 | 78 | 92 | 91 | 87 | 0.830 | 0.354 |
| MMR | 74 | 77 | 83 | 84 | 67 | 56 | 79 | 73 | 67 | 0.518 | 0.278 |
| MR4.5 | 35 | 39 | 50 | 43 | 22 | 22 | 39 | 32 | 33 | 0.644 | 0.659 |
| Best cumulative response, % | |||||||||||
| CCyR | 92 | 100 | 100 | 93 | 78 | 78 | 93 | 91 | 87 | 0.670 | 0.324 |
| MMR | 78 | 85 | 83 | 76 | 67 | 67 | 86 | 86 | 80 | 1.000 | 0.472 |
| MR4.5 | 70 | 69 | 83 | 68 | 56 | 44 | 69 | 64 | 60 | 0.617 | 0.565 |
One patient in each cohort had both renal and liver dysfunction.
Abbreviations: MR 4.5, molecular response with 4.5 log reduction on the international scale; MMR, major molecular response; CCyR, complete cytogenetic response; CHR, complete hematologic response.
At the end of the study period 10 patients had died, including 3 (20%) of 15 patients with pre-existing renal dysfunction, 1 (5%) of 22 patients with pre-existing liver dysfunction, and 7 (4%) of 180 patients with normal organ function (one patient had concomitant renal and liver dysfunction). Two patients with pre-existing renal dysfunction and 1 patient with both pre-existing renal and liver dysfunction that died had been treated with nilotinib. Their cause of death was respiratory failure due to recurrent aspiration pneumonia following extensive surgery for esophageal cancer in one (MR4.5 at the time of death), one died of non-neutropenic sepsis (MMR at time of death), and one, with both renal and liver dysfunction, died of unknown cause after discontinuing TKI due to loss of insurance.
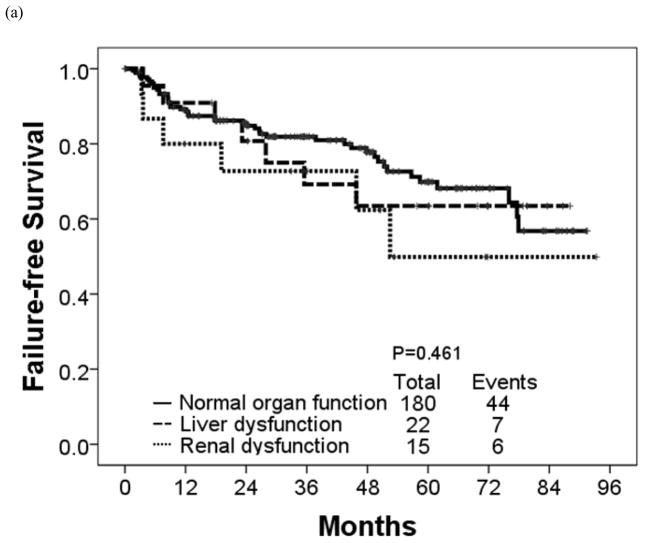
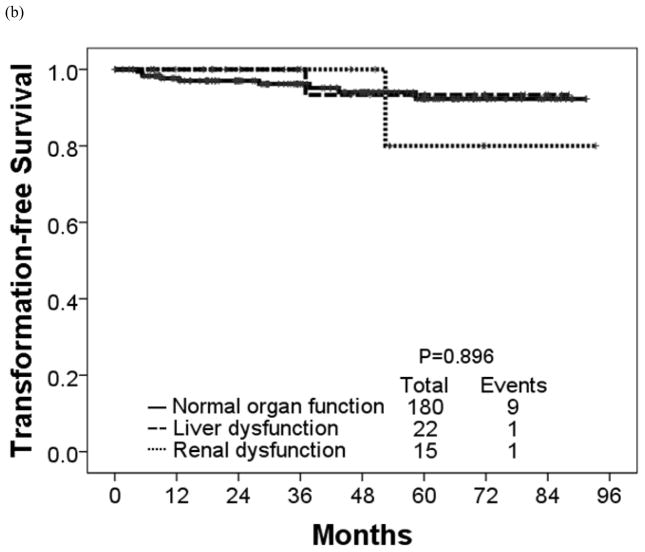
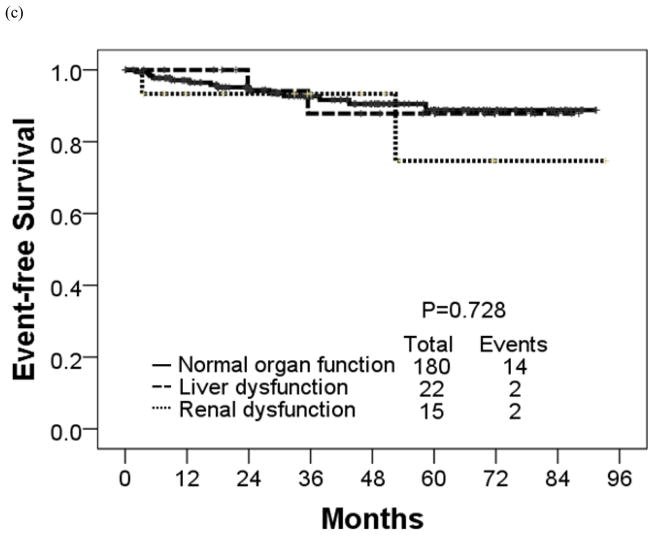
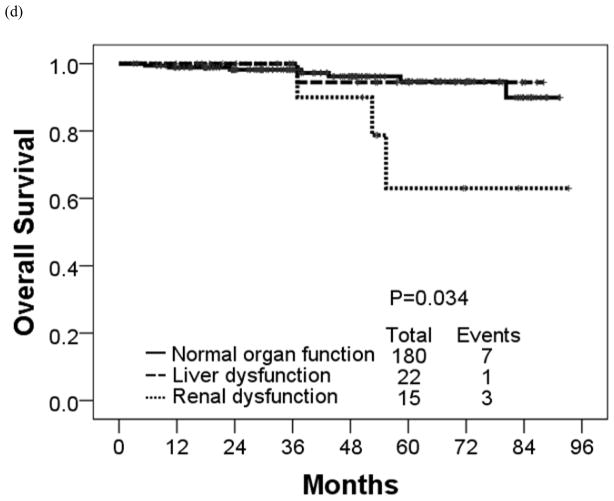
The estimated 4-year OS rate was 90% for patients with renal dysfunction, 94% for patients with liver dysfunction, and 96% for patients with normal organ function (P = 0.034) (Table 7; Figure 3). These 3 groups had similar estimated 4-year EFS rates (93%, 88%, and 91%, respectively) (P = 0.728). The corresponding rates for 4-year FFS were 62%, 63% and 77% in patients with renal dysfunction, liver dysfunction and normal organ function, respectively (P = 0.461) and TFS were 100%, 93%, and 94%, respectively (P = 0.896).
Table 7.
Long-term outcomes for patients treated with dasatinib or nilotinib by pre-existing organ function
| 4-yr survival, % | Dasatinib* N=107 |
Nilotinib* N=108 |
Overall N=215 |
P | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Normal N=89 |
Liver N=13 |
Renal N=6 |
Normal N=91 |
Liver N=9 |
Renal N=9 |
Normal N=180 |
Liver N=22 |
Renal N=15 |
Normal vs. Liver | Normal vs. Renal | |
| FFS | 76 | 73 | 55 | 81 | 52 | 67 | 78 | 63 | 62 | 0.832 | 0.206 |
| TFS | 97 | 90 | 100 | 93 | 100 | 100 | 94 | 93 | 100 | 0.806 | 0.730 |
| EFS | 90 | 91 | 100 | 92 | 83 | 88 | 91 | 88 | 93 | 0.976 | 0.426 |
| OS | 97 | 100 | 100 | 95 | 88 | 83 | 94 | 96 | 90 | 0.886 | 0.012 |
One patient in each cohort had both renal and liver dysfunction.
Abbreviations: FFS, failure free survival; TFS, treatment free survival; EFS, event free survival; OS, overall survival.
Figure 3.
(a) Failure-free, (b) transformation-free, (c) event-free, and (d) overall survival in each organ function group.
Cox Regression Analysis
Multivariate Cox proportional hazards analysis for survival showed age at diagnosis (P <0.001; HR, 1.107; 95% CI, 1.053–1.163), and the achievement of CCyR (P <0.001; HR, 0.037; 95% CI, 0.006–0.235) were associated with survival (Table 8). There was a trend for worse survival among patients with baseline renal dysfunction (P= 0.136; HR, 2.801; 95% CI, 0.723–10.853).
Table 8.
Multivariate Cox proportional hazards analysis for survival
| Univariate | Multivariate Analysis | |||
|---|---|---|---|---|
| P | P | HR | 95% CI | |
| Age at diagnosis | <0.001 | <0.001 | 1.107 | 1.053–1.163 |
| Clonal evolution at diagnosis | 0.702 | |||
| Sokal risk score | 0.582 | |||
| Type of TKIs | 0.108 | |||
| Time from diagnosis to TKI | 0.887 | |||
| Presence of liver dysfunction | 0.639 | |||
| Presence of renal dysfunction | 0.019 | 0.136 | 2.801 | 0.723–10.853 |
| BCR-ABL <10% at 3 months | 0.507 | |||
| Response with a time-varying variable* | ||||
| CCyR | 0.007 | <0.001 | 0.037 | 0.006–0.235 |
| MMR | 0.267 | |||
| MR4.5 | 0.091 | |||
| CMR | 0.450 | |||
Abbreviations: HR, hazard ratio; CI, confidence interval; TKI, tyrosine kinase inhibitor; CCyR, complete cytogenetic response; MMR, major molecular response; MR4.5, molecular response by a 4.5 log reduction on the international scale; CMR, complete molecular response.
each variable was calculated for multivariate analysis separately.
Discussion
Our analysis suggests that nilotinib and dasatinib elicit similar response rates and tolerability among CML-CP patients with or without pre-existing liver and/or renal dysfunction. We had previously shown that imatinib therapy is generally safe and effective in patients with CML-CP and pre-existing liver or renal dysfunction albeit with more frequent dose reductions.22 No such data had been previously published with dasatinib and nilotinib. The DASISION and ENESTnd trials excluded patients with pre-existing liver or renal dysfunction. Inclusion criteria for DASISION included adequate hepatic function defined as total bilirubin ≤2.0 times ULN, ALT and AST ≤2.5 times ULN, and adequate renal function defined as serum creatinine ≤3x ULN. The inclusion criteria for ENESTnd included aminotransferases, bilirubin, and creatinine that were no higher than 1.5x ULN. To our knowledge, this is the first study to describe the efficacy and safety of nilotinib and dasatinib in patients with pre-existing renal or liver dysfunction compared to those with normal organ function.
The liver toxicity of TKIs is well recognized, with most of them having a low but significant incidence of liver AEs, including single-digit rates of grade 3/4, occasionally leading to treatment discontinuation. The renal toxicity of these agents is less well recognized. We recently reported that over the course of therapy there is a decline in GFR among patients treated with imatinib, but not among those treated with dasatinib or nilotinib.23 Increased events of acute kidney injury were observed in patients with pre-existing renal dysfunction. These events were mostly reversible with hydration or transient cessation of TKIs. Since nilotinib and its metabolite are not renally excreted, and less than 4% of dasatinib and its metabolite are renally excreted, a decrease in renal clearance due to modestly impaired renal function is not expected according to the prescribing information. 24, 25 It is important to underscore that despite the general safety of dasatinib and nilotinib in this analysis among patients with mild to moderate renal dysfunction receiving these agents as initial therapy for CML, 9 of the 15 (60%) patients with renal dysfunction had some worsening of renal function. Thus, patients with renal dysfunction need closer monitoring of renal function for dose adjustment or transient cessation.
Patients with mild baseline liver dysfunction tolerated the standard dose of nilotinib and dasatinib without increased incidence of adverse events. Pharmacokinetic analysis of dasatinib in patients with Child-Pugh class B or C liver function compared to patients with normal liver function showed that patients with Child-Pugh class B had decrease in dose-normalized Cmax and Area Under the Curve (AUC) by 47% and 8%, respectively, and patients with Child-Pugh class C by 43% and 28%, respectively. The difference in Cmax and AUC were not clinically relevant, and dose adjustments were not recommended. 25 Pharmacokinetic studies of nilotinib showed the mean AUC values were increased on average of 35%, 35%, and 56% in patients with Child-Pugh class A, B, and C, respectively. Therefore, a lower starting dose of nilotinib is recommended in patients with hepatic impairment. 24 Patients with liver dysfunction receiving TKI might need closer monitoring of liver function tests for toxicity monitoring.
Interestingly, two patients treated with nilotinib in the cohort with renal dysfunction experienced gastrointestinal hemorrhage leading to treatment discontinuation. Dasatinib is associated with increased risk of bleeding among CML-CP patients in the absence of thrombocytopenia. Dasatinib inhibits platelet function by impairing arachidonic acid–and epinephrine-induced aggregation, but nilotinib has no detectable inhibitory activity.26 However, the ENESTnd trial reported increased incidence of hemorrhage. Grade 3 or 4 hemorrhage were observed in 3% of patients in the nilotinib 300 mg twice daily group, 4% in the nilotinib 400 mg twice daily group compared to 1% in the imatinib group.7 In our study, two nilotinib-treated patients with renal dysfunction had grade 3 or 4 bleeding, both from gastric ulcers. There is no clear evidence that nilotinib contributed to the bleeding in these patients.
Notwithstanding the limitations conferred by the small subsets of patients with organ dysfunction in the present study, we found no significant differences in the incidence of most nonhematologic toxicities between patients with normal organ function and patients with liver or renal organ dysfunction. Most patients with mild liver or renal dysfunction can be safely treated with nilotinib or dasatinib and can have an outcome equally favorable as those with no organ dysfunction.
There are several limitations in this study. First, the retrospective nature of this analysis potentially influenced some of the conclusions drawn from its findings. However, although the analysis was retrospective, the patients included in the analysis had been treated in prospective studies in which data were collected in real time. In addition, because these patients were treated in clinical trials, they were followed at specific interval, with frequent monitoring of the efficacy and safety of the agents. Second, there are small numbers of patients in each organ dysfunction group, and most patients had only mild renal or liver organ dysfunction. However, previous studies did not assess patients with organ dysfunction in a prospective fashion making THE information presented here valuable for the prescribing physician facing this clinical scenario. Importantly, this data should not be extrapolated to patients with more significant renal or liver dysfunction not represented in these cohorts. Additional studies are required to define the proper management of patients with moderate to severe organ dysfunction.
In conclusion, nilotinib and dasatinib are effective therapies for patients with CML-CP who present with pre-existing modest liver or renal dysfunction. However, patients with pre-existing renal dysfunction who are treated with dasatinib or nilotinib have an increased risk of acute kidney injury, and patients with pre-existing renal dysfunction who are treated with nilotinib may be at an increased risk of bleeding. Nonetheless, most patients with mild liver dysfunction or mild or moderate renal dysfunction can be safely treated with dasatinib or nilotinib. Further studies to assess the safety and efficacy of dasatinib and nilotinib are needed for patients with more significant baseline organ dysfunction.
Acknowledgments
This study was supported in part by the MD Anderson Cancer Center Support Grant CA016672 and Award Number P01 CA049639 from the National Cancer Institute.
Footnotes
Authorship contributions
K.S. treated the patients, collected data, designed the study, analyzed the data, and wrote the manuscript. J.C. treated the patients, designed the study, analyzed the data and edited the manuscript. A.L. designed the study, analyzed the data and edited the manuscript. P.J., treated the patients and collected the data. S.P. managed the data. S.O., E.J., G.B., N,P., N.D., T.K., A.F., H.K. treated the patients. All authors provided significant intellectual input, and reviewed and approved the final version of the manuscript.
Conflicts of interests
J.C. received research support from Ariad, BMS, Novartis, Pfizer, and Teva, and is a consultant for Ariad, BMS, Novartis and Pfizer. H.K. received research grants from Novaris, BMS, Pfizer, Ariad. E.J. received consultancy for Ariad, BMS, TEVA, and Pfizer. F.R. received research funding from Novartis and BMS. Other authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Golemovic M, Verstovsek S, Giles F, et al. AMN107, a novel aminopyrimidine inhibitor of Bcr-Abl, has in vitro activity against imatinib-resistant chronic myeloid leukemia. Clin Cancer Res. 2005;11:4941–4947. doi: 10.1158/1078-0432.CCR-04-2601. [DOI] [PubMed] [Google Scholar]
- 2.Weisberg E, Manley PW, Breitenstein W, et al. Characterization of AMN107, a selective inhibitor of native and mutant Bcr-Abl. Cancer Cell. 2005;7:129–141. doi: 10.1016/j.ccr.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 3.Shah NP, Tran C, Lee FY, Chen P, Norris D, Sawyers CL. Overriding imatinib resistance with a novel ABL kinase inhibitor. Science. 2004;305:399–401. doi: 10.1126/science.1099480. [DOI] [PubMed] [Google Scholar]
- 4.Cortes JE, Jones D, O'Brien S, et al. Nilotinib as front-line treatment for patients with chronic myeloid leukemia in early chronic phase. J Clin Oncol. 2010;28:392–397. doi: 10.1200/JCO.2009.25.4896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosti G, Palandri F, Castagnetti F, et al. Nilotinib for the frontline treatment of Ph(+) chronic myeloid leukemia. Blood. 2009;114:4933–4938. doi: 10.1182/blood-2009-07-232595. [DOI] [PubMed] [Google Scholar]
- 6.Saglio G, Kim DW, Issaragrisil S, et al. Nilotinib versus imatinib for newly diagnosed chronic myeloid leukemia. N Engl J Med. 2010;362:2251–2259. doi: 10.1056/NEJMoa0912614. [DOI] [PubMed] [Google Scholar]
- 7.Kantarjian HM, Hochhaus A, Saglio G, et al. Nilotinib versus imatinib for the treatment of patients with newly diagnosed chronic phase, Philadelphia chromosome-positive, chronic myeloid leukaemia: 24-month minimum follow-up of the phase 3 randomised ENESTnd trial. Lancet Oncol. 2011;12:841–851. doi: 10.1016/S1470-2045(11)70201-7. [DOI] [PubMed] [Google Scholar]
- 8.Larson RA, Hochhaus A, Hughes TP, et al. Nilotinib vs imatinib in patients with newly diagnosed Philadelphia chromosome-positive chronic myeloid leukemia in chronic phase: ENESTnd 3-year follow-up. Leukemia. 2012;26:2197–2203. doi: 10.1038/leu.2012.134. [DOI] [PubMed] [Google Scholar]
- 9.Kantarjian HM, Shah NP, Cortes JE, et al. Dasatinib or imatinib in newly diagnosed chronic-phase chronic myeloid leukemia: 2-year follow-up from a randomized phase 3 trial (DASISION) Blood. 2012;119:1123–1129. doi: 10.1182/blood-2011-08-376087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kantarjian H, Shah NP, Hochhaus A, et al. Dasatinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2010;362:2260–2270. doi: 10.1056/NEJMoa1002315. [DOI] [PubMed] [Google Scholar]
- 11.Radich JP, Kopecky KJ, Appelbaum FR, et al. A randomized trial of dasatinib 100 mg versus imatinib 400 mg in newly diagnosed chronic-phase chronic myeloid leukemia. Blood. 2012;120:3898–3905. doi: 10.1182/blood-2012-02-410688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jain P, Kantarjian H, Alattar ML, et al. Long-term molecular and cytogenetic response and survival outcomes with imatinib 400 mg, imatinib 800 mg, dasatinib, and nilotinib in patients with chronic-phase chronic myeloid leukaemia: retrospective analysis of patient data from five clinical trials. The Lancet Haematology. 2:e118–e128. doi: 10.1016/S2352-3026(15)00021-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sasaki K, Strom SS, O'Brien S, et al. Relative survival in patients with chronic-phase chronic myeloid leukaemia in the tyrosine-kinase inhibitor era: analysis of patient data from six prospective clinical trials. The Lancet Haematology. 2:e186–e193. doi: 10.1016/S2352-3026(15)00048-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cortes JE, Jones D, O'Brien S, et al. Results of dasatinib therapy in patients with early chronic-phase chronic myeloid leukemia. J Clin Oncol. 2010;28:398–404. doi: 10.1200/JCO.2009.25.4920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Di Gion P, Kanefendt F, Lindauer A, et al. Clinical pharmacokinetics of tyrosine kinase inhibitors: focus on pyrimidines, pyridines and pyrroles. Clin Pharmacokinet. 2011;50:551–603. doi: 10.2165/11593320-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 16.Christopher LJ, Cui D, Wu C, et al. Metabolism and disposition of dasatinib after oral administration to humans. Drug Metab Dispos. 2008;36:1357–1364. doi: 10.1124/dmd.107.018267. [DOI] [PubMed] [Google Scholar]
- 17.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 18.Druker BJ, Guilhot F, O'Brien SG, et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355:2408–2417. doi: 10.1056/NEJMoa062867. [DOI] [PubMed] [Google Scholar]
- 19.Kantarjian H, Sawyers C, Hochhaus A, et al. Hematologic and cytogenetic responses to imatinib mesylate in chronic myelogenous leukemia. N Engl J Med. 2002;346:645–652. doi: 10.1056/NEJMoa011573. [DOI] [PubMed] [Google Scholar]
- 20.Sokal JE, Cox EB, Baccarani M, et al. Prognostic discrimination in “good-risk” chronic granulocytic leukemia. Blood. 1984;63:789–799. [PubMed] [Google Scholar]
- 21.Kantarjian HM, Keating MJ, Smith TL, Talpaz M, McCredie KB. Proposal for a simple synthesis prognostic staging system in chronic myelogenous leukemia. Am J Med. 1990;88:1–8. doi: 10.1016/0002-9343(90)90119-x. [DOI] [PubMed] [Google Scholar]
- 22.Tong WG, Kantarjian H, O'Brien S, et al. Imatinib front-line therapy is safe and effective in patients with chronic myelogenous leukemia with pre-existing liver and/or renal dysfunction. Cancer. 2010;116:3152–3159. doi: 10.1002/cncr.25071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yilmaz M, Kantarjian HM, Quintas-Cardama A, et al. Estimated Glomerular Filtration Rate Changes In Patients (Pts) With Chronic Myeloid Leukemia (CML) Treated With Tyrosine Kinase Inhibitors (TKI) 2013 doi: 10.1002/cncr.29587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tasigna (nilotinib) [prescribing information] East Hanover, NJ: Novartis; Jan, 2015. [Google Scholar]
- 25.Sprycel (dasatinib) [prescribing information] Princeton, NJ: Bristol-Myers Squibb Company; Apr, 2014. [Google Scholar]
- 26.Quintas-Cardama A, Han X, Kantarjian H, Cortes J. Tyrosine kinase inhibitor-induced platelet dysfunction in patients with chronic myeloid leukemia. Blood. 2009;114:261–263. doi: 10.1182/blood-2008-09-180604. [DOI] [PMC free article] [PubMed] [Google Scholar]