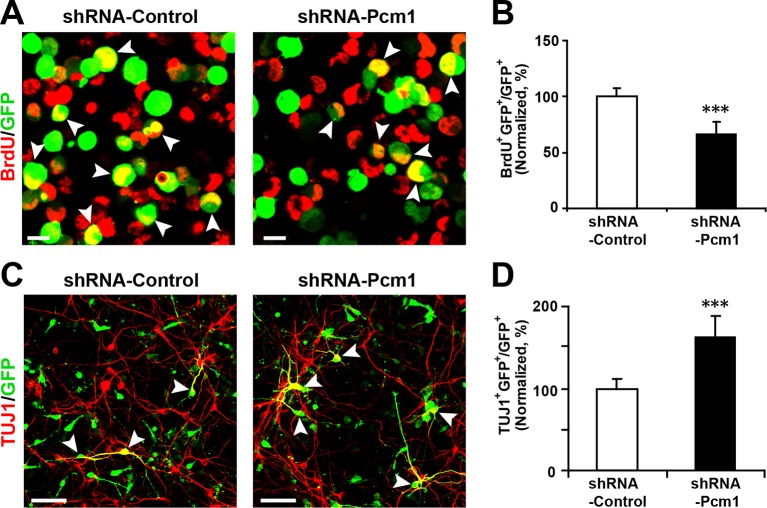
Figure 5. PCM1 knockdown decreases NPC neurogenesis.
(A,B) PCM1 knockdown in NPCs decreases neural proliferation. NPCs were electroporated with a plasmid expressing PCM1 shRNA or with a control vector and pulse-labeled with BrdU for 6 hr. NPCs were immunostained with an antibody against BrdU. The arrowheads indicate BrdU and GFP double-positive cells. Scale bars, 10 µm. Quantification of the number of GFP-BrdU double-positive cells relative to the total number of GFP-positive cells (B). (C,D) PCM1 knockdown in NPCs increases neurogenesis. NPCs were electroporated with a plasmid expressing PCM1 shRNA or with a control vector and then immunostained with antibodies against TUJ1. The arrowheads indicate TUJ1+GFP+ cells. Scale bars, 50 µm. Quantification of the number of GFP-TUJ1 double-positive cells relative to the number of GFP-positive cells (D). More than 1500 GFP-positive cells were counted for each condition. At least three sets of independent experiments were performed. The values represent the mean ± s.d. (n = 3). Student’s t-test, differences were considered significant at ***p<0.001.
Figure 5—figure supplement 1. Efficient knockdown of PCM1.
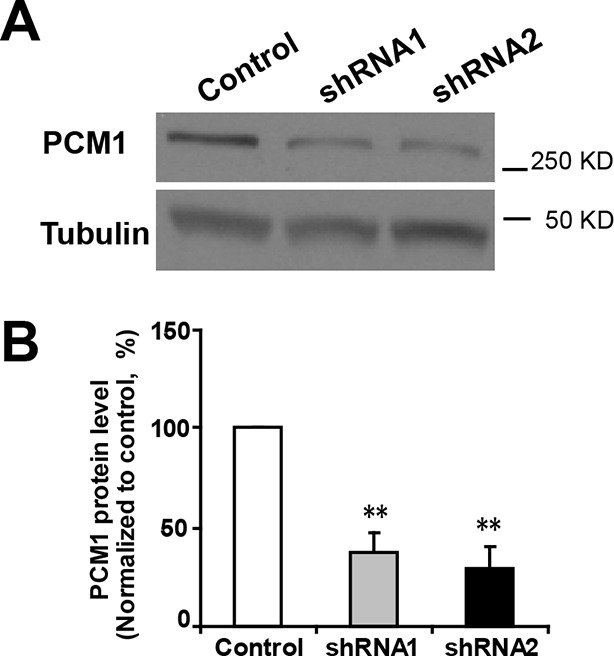
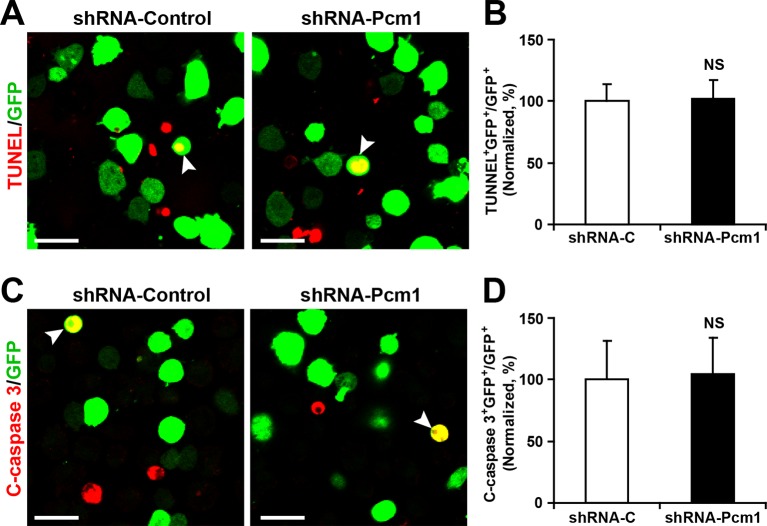
Figure 5—figure supplement 2. PCM1 knockdown in NPCs did not trigger apoptotic cell death.
Figure 5—figure supplement 3. PCM1 knockdown in NPCs increases neurogenesis.
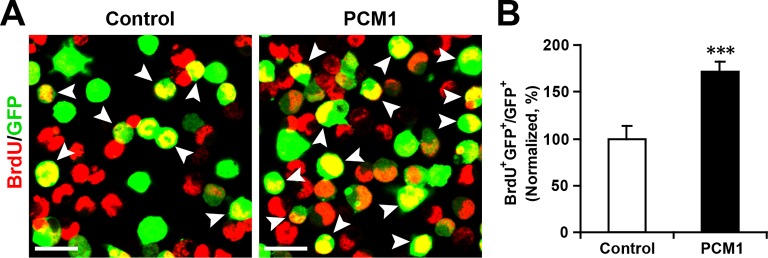
Figure 5—figure supplement 4. Overexpression of PCM1 increases NPC proliferation.
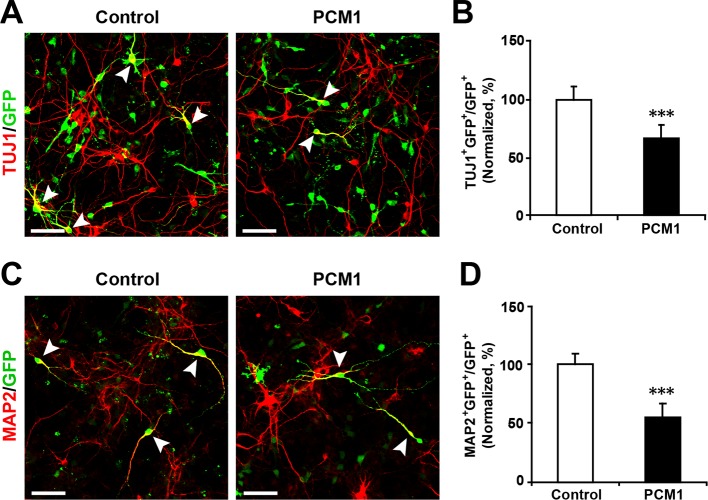
Figure 5—figure supplement 5. Overexpression of PCM1 decreases NPC neuronal differentiation.
Figure 5—figure supplement 6. Overexpression of PCM1 in NPCs did not trigger apoptotic cell death.
Figure 5—figure supplement 7. PCM1 regulates NPC proliferation and differentiation in vivo.