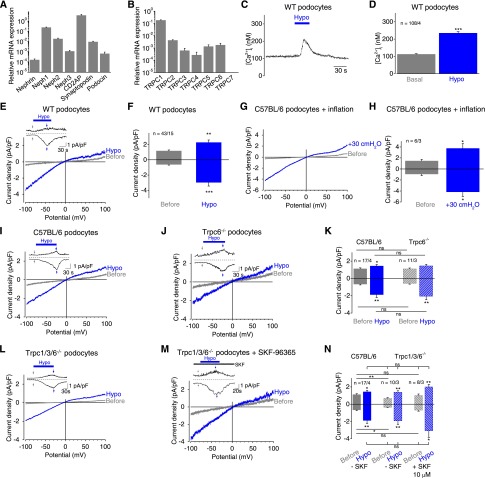
Figure 1.
Mechanical membrane stretch induces inwardly rectifying currents and intracellular calcium increases in podocytes. (A and B) Relative mRNA expression levels of selected podocyte marker proteins (A) and TRPC channels (B) in primary podocytes determined by quantitative PCR analysis from three independent experiments. (C) Exemplary time courses of the intracellular calcium concentration [Ca2+]i of fura-2–loaded podocytes. Application of hypoosmotic bath solution “Hypo” is indicated. (D) Summary of [Ca2+]i before (gray bar) and during (blue bar) application of hypoosmotic bath solution. Numbers indicate the numbers of measured cells and of independent experiments. (E–N) Electrophysiologic whole-cell measurements of wild-type (WT) (E), C57BL/6 (G and I), TRPC6 gene-deficient (Trpc6−/−) (J), and TRPC1/3/6 gene-deficient (Trpc1/3/6−/−) (L and M) podocytes in the presence (M) or absence (L) of the nonselective TRPC blocker SKF-96365 with exemplary current density voltage (CDV) relationships. CDVs are displayed before (gray) and during (blue) application of hypoosmotic bath solution “Hypo” and of positive pipette pressure of 30 cmH2O “inflation” (G). Insets show current density time courses at holding potentials of ±100 mV. Stippled lines represent zero current. Blue bars indicate application of hypoosmotic solution. Arrows represent the time points of depicted CDV traces. (F, H, K, and N) Summary of current densities before (gray and gray hatched bars) and during application of hypoosmotic bath solution or of positive pipette pressure (blue and blue hatched bars) of respective control (solid bars) and gene-deficient podocytes (hatched bars) at ±100 mV. Numbers display the numbers of measured cells and of independent experiments. Nonsignificant (ns) differences are indicated. *P<0.05; **P<0.01; ***P<0.001; ns P>0.05.