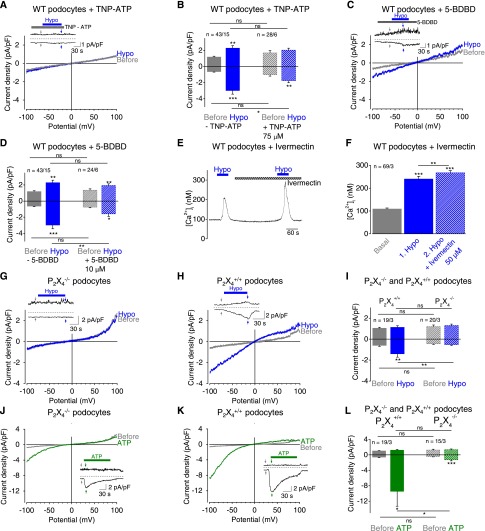
Figure 4.
P2X4 channels in podocytes are activated upon mechanical stimulation. (A–D) Electrophysiologic whole-cell measurements of podocytes. (A and C) Exemplary CDV relationships before (gray) and during (blue) application of hypoosmotic solution in the presence of 75 µM TNP-ATP (A) and of 10 µM 5-BDBD (C). Insets show current density time courses at ±100 mV. Stippled lines represent zero current. Applications of hypoosmotic solution and of TNP-ATP (A) or of 5-BDBD (C) are indicated. Arrows represent the time points of depicted CDV traces. (B and D) Summaries of current densities before (gray and gray hatched bars) and during application of hypoosmotic solution (blue and blue hatched bars) in the presence and absence of TNP-ATP (B) or of 5-BDBD (D) determined at holding potentials of ±100 mV. (E and F) Fluorimetric calcium measurements with fura-2–loaded podocytes. (E) Exemplary time courses of [Ca2+]i and applications of hypoosmotic solution and of 50 µM ivermectin are indicated. (F) Summary of [Ca2+]i before (gray bar) and during application of hypoosmotic solution in the presence (blue hatched bar) and absence (blue bar) of ivermectin. Numbers indicate the numbers of measured cells and of independent experiments. (G–L) Electrophysiologic whole-cell measurements of P2X4−/− and of P2X4+/+ control podocytes. (G, H, J, K) Exemplary CDV relationships of P2X4−/− (G and J) and of P2X4+/+ control podocytes (H and K) before (gray) and during application of hypoosmotic solution (blue) or of 100 µM ATP (green). Insets show current density time courses at ±100 mV. Stippled lines represent zero current. Application of hypoosmotic solution and of ATP is indicated. Arrows represent the time points of depicted CDV traces. (I and L) Summaries of current densities before (gray and gray hatched bars) and during application of hypoosmotic solution (blue and blue hatched bars) (I) or of 100 µM ATP (green and green hatched bars) (L) of P2X4−/− and P2X4+/+ control podocytes determined at ±100 mV. Numbers indicate the numbers of measured cells and of independent experiments. Nonsignificant differences (ns) are indicated. *P<0.05; **P<0.01; ***P<0.001; ns P>0.05.