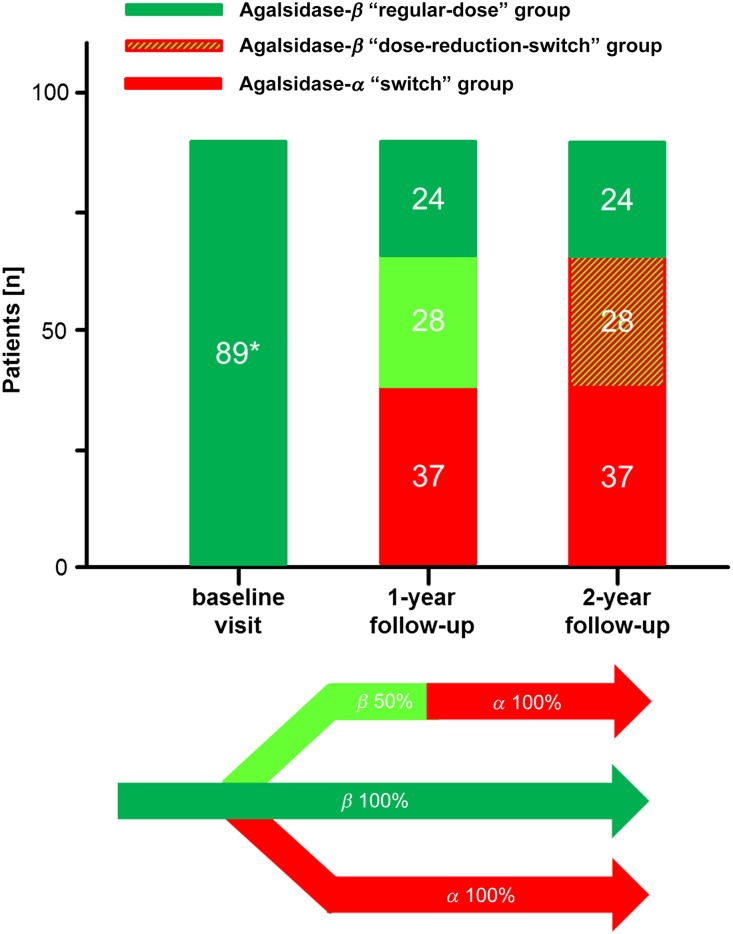
Figure 1.
General overview of the study population. All patients were at least 12 months under a stable dose of agalsidase-β (1.0 mg/kg e.o.w.). After the baseline visit, the treatment strategy for the following years was chosen by a FD specialist physician team and the patients. Because of ongoing supply shortage, patients under agalsidase-β reduction (0.5 mg/kg e.o.w.) were switched after 12 months to agalsidase-α (0.2 mg/kg e.o.w.). *Patients were at least 12 months under a regular-dose of agalsidase-β.