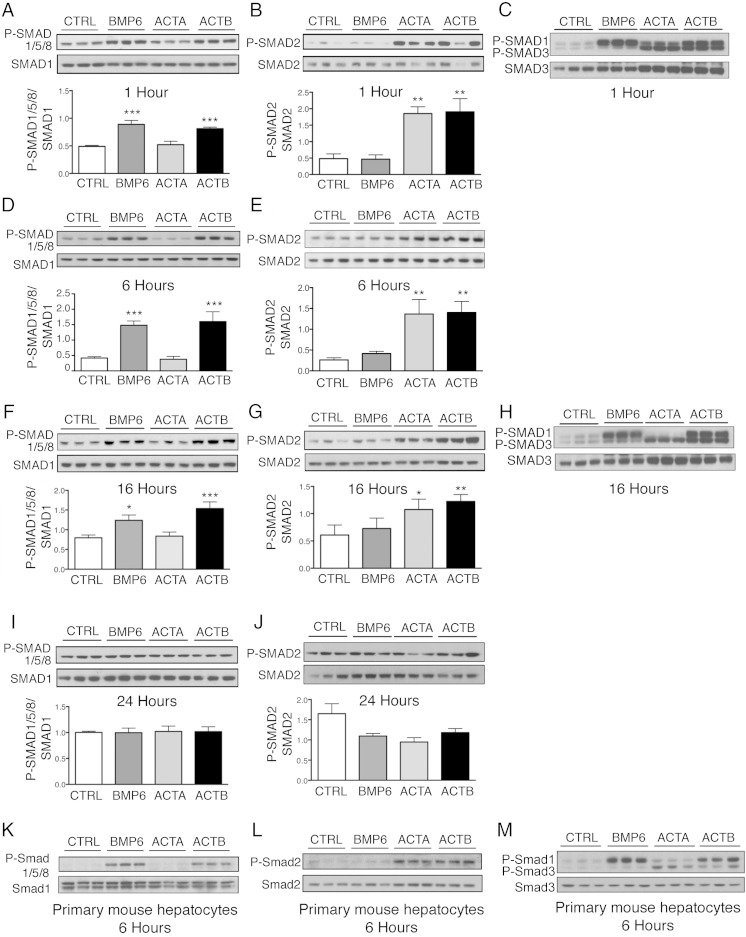
Figure 2.
Activin B (ACTB) stimulates SMAD1/5/8 phosphorylation with similar kinetics and potency as BMP6, and SMAD2/3 phosphorylation with similar kinetics and potency as activin A (ACTA) in Hep3B cells and primary mouse hepatocytes. Hep3B cells (A–J) and primary mouse hepatocytes (K–M) were treated without (CTRL) or with 5-ng/mL BMP6, ACTA, or ACTB and analyzed at 1, 6, 16, and 24 hours as indicated for P-SMAD1/5/8 relative to total SMAD1 (A, D, F, I, and K) and for P-SMAD2 relative to total SMAD2 (B, E, G, J, and L) by immunoblot and chemiluminescence quantification. Chemiluminescence results are reported as mean ± SEM of 3 separate experiments (P-SMAD1/5/8) or 2 separate experiment (P-SMAD2) each performed in triplicate. Representative immunoblots are shown. Statistical significance was determined by one-way ANOVA with Dunnett's post hoc test (*, P < .05; **, P < .01; ***, P < .001 relative to CTRL). Activation of the P-SMAD2/3 and P-SMAD1/5/8 pathways was also verified by immunoblot using a P-SMAD3 antibody that recognizes both P-SMAD3 and P-SMAD1 as indicated (C, H, and M). Chemiluminescence quantitation was not performed on primary hepatocyte samples because CTRL bands were too faint, nor on P-SMAD3/1 gels because SMAD3 and SMAD1 bands were too close to each other.