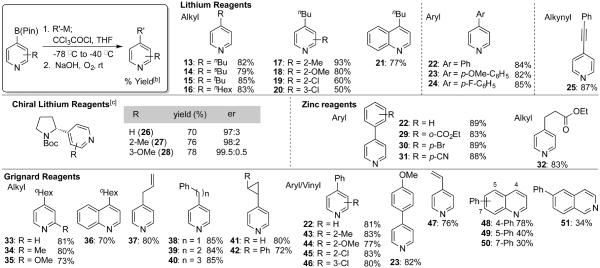
Table 1.
Substrate scope for preparation of substituted pyridines[a]
Typical conditions: het-B(pin) (1 equiv., 0.3 mmol), R-M (1.1 equiv.), −78 °C, CCI3COCI (2 equiv.), −78 to −40 °C; 10% NaOH aq, rt, O2 balloon.
Isolated yield.
Typical conditions: (−)sparteine (1.3 equiv., 0.39 mmol), sBuLi (1.3 equiv., 0.39 mmol), N-Boc pyrrolidine (1.3 equiv., 0.39 mmol); het-B(pin) (1 equiv., 0.3 mmol), −78 °C, CCI3COCI (3.5 equiv.), −78 °C; saturated aq. Na2CO3 rt, O2 balloon. het-B(pin) = heteroaryl pinacol bononic ester.