Abstract
Social approval is a reward that uses abstract social reinforcers to guide interpersonal interactions. Few studies have specifically explored social reward processing and its related neural substrates in schizophrenia. Fifteen patients with schizophrenia and fifteen healthy controls participated in a two-part study to explore the functional neural correlates of social approval. In the first session, participants were led to believe their personality would be assessed based on their results from various questionnaires and an interview. Participants were then presented with the results of their supposed evaluation in the scanner, while engaging in a relevant fMRI social approval task. Subjects provided subjective reports of pleasure associated with receiving self-directed positive or negative feedback. Higher activation of the right parietal lobe was found in controls compared with individuals with schizophrenia. Both groups rated traits from the high social reward condition as more pleasurable than the low social reward condition, while intergroup differences emerged in the low social reward condition. Positive correlations were found in patients only between subjective ratings of positive feedback and right insula activation, and a relevant behavioural measure. Evidence suggests potential neural substrates underlying the cognitive representation of social reputation in schizophrenia.
Keywords: schizophrenia, social reward, fMRI, self-processing, social cognition
Introduction
Social reward processing utilizes abstract reinforcers to guide appropriate motivated behaviours in social activities. For instance, individuals strive towards establishing a good reputation among their peers (Benabou and Tirole, 2006). The recognition that one has a good reputation can induce a feeling of happiness (i.e. a hedonic component of reward), and individuals are often motivated to seek such social approval (i.e. a motivational component of reward) (Bateson et al., 2006; Kurzban and Aktipis, 2007). Social approval is a key social reward that has a profound impact on everyday social decision making (Leary 2007; Boksem et al., 2011).
Few studies have investigated this type of social reward in schizophrenia. Patients with schizophrenia often present difficulties when engaging in reciprocal relationships (Bellack et al., 1990; Galderisi et al., 2014). It has been argued that poor social functioning in patients may be strongly related to social cognitive deficits, including an altered ability to process positive feedback from others (Pinkham et al., 2003; Cohen et al., 2011). Past studies exploring reward processing in schizophrenia have primarily made use of non-social stimuli, such as money, positive pictures and juice (Gard et al., 2007; Diekhof et al., 2008; Gold et al., 2008; Waltz et al., 2009, Waltz et al., 2010). Results indicate that patients have reasonably intact hedonic responses, at least for simple non-social stimuli, but impaired motivation and reward representation, leading to failure in directing goal-oriented behaviour (Horan et al., 2006; Barch, 2008; Barch and Dowd, 2010).
At the neural level, several aberrant cortical-striatal interactions have been highlighted in schizophrenia during both the anticipation and delivery of non-social reward (Waltz et al., 2009; Barbour et al., 2010; Simon et al., 2010; Gradin et al., 2013; Strauss et al., 2014). It has previously been shown that patients have a reduced response compared with healthy controls in reward-related brain regions (e.g., ventral striatum) upon presentation of a clear positive non-social stimulus (Murray et al., 2008; Koch et al., 2010). It is still unclear, however, whether these patterns of brain abnormalities observed in the context of non-social reward processing applies to more abstract and complex social rewards. There are some indications suggesting that reward-processing deficits in schizophrenia may be even more pronounced for abstract social rewards, such as holding a good reputation in social circles (i.e. social approval) (Penn et al., 1997). Gold et al. (2008) examined results from several studies all converging to the idea that individuals with schizophrenia exhibit deficits in various aspects of the reward system, including a reduced ability to use subjective reward experiences to guide short- and long-term learning. These neurocognitive deficits can have significant implications for functional outcome in schizophrenia, with strong evidence for social cognition as a mediator of this relationship (Brekke et al., 2005; Couture et al., 2006; Gard et al., 2009).
In an innovative study conducted on healthy controls, Izuma et al. (2008) implemented a functional magnetic resonance imaging (fMRI) paradigm to elucidate the neural correlates of social approval. Their experimental design led participants to believe they were being evaluated by a group of peers, based on their responses on several questionnaires and during an interview. Personality traits were supposedly selected by the evaluators to describe participants' personality and subsequently presented to participants in the scanner. Social reward, specifically social approval, was defined as the positive personality traits representing participants' good reputation. In reality the level of social approval was systematically manipulated. Their results suggested that social approval activates similar brain regions associated with processing of concrete non-social rewards (e.g., monetary gains) encompassing the left caudate nucleus and putamen of the striatum. In addition, increased activity in the medial prefrontal cortex (mPFC) was noted, which is consistent with this higher cortical region’s established function in self-referential processing (Northoff and Bermpohl, 2004; Moran et al., 2006; Northoff et al., 2006; Harvey et al., 2007; van der Meer et al., 2010).
This study is the first to examine the neural correlates of social approval in the context of schizophrenia. Specifically, the study aims to expand on the experimental design previously defined by Izuma et al., and implement an effective social approval paradigm to assess how individuals with schizophrenia respond to receiving feedback about the self. At the behavioural level, we expect schizophrenia patients to report lower levels of pleasure when receiving positive feedback about their personality. At the neural level, it is expected that patients with schizophrenia will have a reduced response in reward-related regions, particularly within the ventral striatum, upon presentation of positive feedback directed towards them. In addition, it is hypothesized that patients will show a deficit in processing their own reputation, as revealed by decreased mPFC activation.
Methods
Participants
The reported analyses are based on data collected from 15 right-handed patients with schizophrenia (11 males, mean age ± SD = 33.1 ± 7.7) and 15 right-handed healthy controls (9 males, mean age ± SD = 35.2 ± 9.8), in the age range of 20–51 years. All subjects had normal vision and were physically healthy, based on medical history. Patients with schizophrenia were recruited from several outpatient clinics affiliated with the Douglas Mental Health University Institute. Controls were recruited from online advertisements. Diagnosis of schizophrenia in the patient group was established using the Structured Clinical Interview for DSM-IV (SCID) (First et al., 1996) conducted by trained clinicians. Positive and negative symptoms of schizophrenia were assessed using the Scale for the Assessment of Positive Symptoms (Andreasen, 1984a) and the Scale for the Assessment of Negative Symptoms (Andreasen, 1984b). Controls were assessed using the SCID-II (First et al., 1997), in addition to the SCID-I. Exclusion criteria for both patients and controls included a report of substance abuse or dependence within one month prior to the assessment, an intelligent quotient (I.Q.) of <70 points as outlined in the Weschler Abbreviated Scale of Intelligence (WASI; Hays et al., 2002), history of a loss of consciousness for more than 10 minutes, a distinguishable neurologic disorder or history of a heritable neurological condition, and/or possible contraindication for the MRI scan. In addition, exclusion criteria for controls included a history of schizophrenia or another psychotic disorder (including both personal and/or first degree relative history), bipolar disorder, or recurrent depressive episodes. Possibility of current depression was assessed with the Calgary Depression Scale (CDS; Addington et al., 1994); additionally, the Hamilton Anxiety Scale (HAS; Hamilton, 1959) was used to evaluate current levels of anxiety. All participants provided written informed consent, and the study was approved by the Douglas Institute institutional review board.
Experimental design
Subjects were asked to participate in two study sessions: the self-introduction phase, followed by the social approval fMRI task. Participants were told the primary aims of the study were to examine personality, where questionnaires and a short interview would be administered and later evaluated by a panel of three clinicians. In fact, the participants’ personality would never be assessed, but this deception was necessary for the social reward component in the MRI scanner. These two sessions were separated by ∼2 weeks, which aided in convincing participants that during this time, the aforementioned ‘clinicians’ would convene and compile a list of personality traits that they felt best suited the participant based on their self-report questionnaires and video recording. The self-introduction phase lasted 3–4 hours in duration, and included a clinical and cognitive evaluation. Any pertinent information on medication, overall physical health, and factors that could potentially be contraindicatory for the MRI session were collected through an additional screening measure. I.Q. scores were estimated using the WASI, which assesses verbal and spatial reasoning and gives rise to Verbal, Performance, and Full-Scale I.Q. scores (Hays et al., 2002). After screening and clinical assessment measures, all participants were required to complete the following questionnaires, to effectively persuade the participants they were participating in a personality study: the Self-Esteem Rating Scale (SERS; Nugent and Thomas, 1993), the Social Desirability Scale (SDS; Crowne and Marlowe, 1960), the Empathy Quotient (EQ; Baron-Cohen and Wheelwright, 2004), the Millon Clinical Multiaxial Inventory (Millon et al., 1997), the Ambiguous Intentions Hostility Questionnaire (Combs et al., 2007), the Social and Physical Anhedonia Scales (Chapman et al., 1976), the Interpersonal Reactivity Index (Davis, 1980), and the Temporal Experience of Pleasure Scale (TEPS; Gard et al., 2006). In addition, all subjects participated in a short video-recorded interview, consisting of open-ended questions to engage the participant in telling the interviewer about their personality, their future goals and plans, and their opinion on a relevant current event. The participants were told the results of their personality evaluation would be revealed to them in the subsequent scanner session. In addition, participants were informed they would also see the evaluation of another person they had not met before in the scanner, where it was emphasized that this was required to reliably assess ‘self-processing’ of one’s personality.
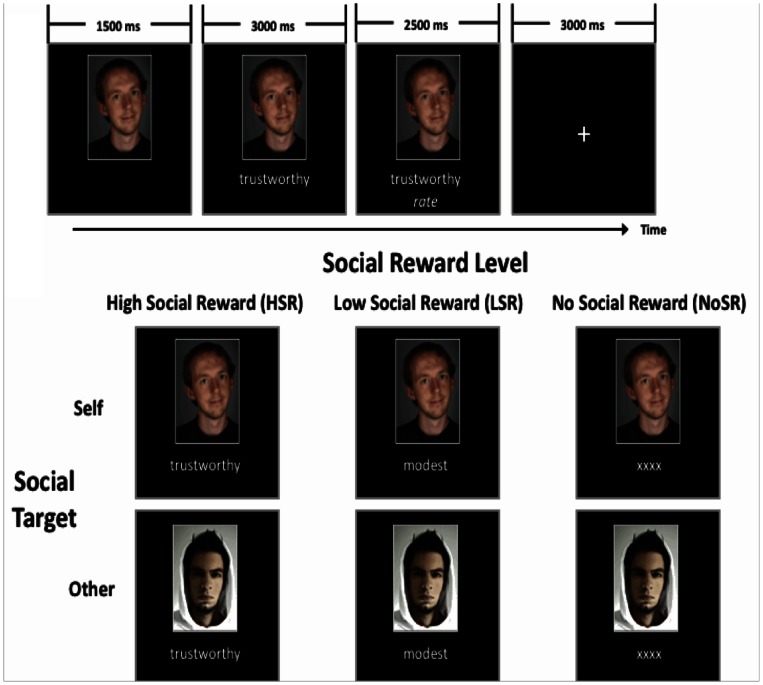
The social approval task administered in the scanner was an event-related 2 × 3 design, with three social reward levels (High Social Reward [HSR], Low Social Reward [LSR], and No Social Reward [NoSR]) and two social target conditions (‘self’ or ‘other’). Stimuli consisted of 120 personality traits taken from Andersen’s published list, composed of 60 HSR and 60 LSR traits, where the reward value of the chosen traits has been previously established (Andersen, 1968). HSR words were highly positive (e.g., intelligent, loyal), whereas LSR words ranged from moderately positive (e.g., modest) to slightly negative (e.g., careless). Because LSR words were globally perceived as negative, this condition will be further referred to as consisting of ‘negative’ feedback. The NoSR condition was defined by the presentation of ‘XXXX’ instead of an adjective. The HSR and LSR conditions were further broken down to create two lists for each condition, resulting in four lists of 30 adjectives each (HSR List A, HSR List B, LSR List A, LSR List B). These four lists were important in establishing the ‘self’ and ‘other’ conditions. H/LSR Lists A were used to represent the subject’s presumable evaluation, whereas H/LSR Lists B represented the evaluation of an unknown ‘other’ person. Lists A and B were switched for an alternative version of the task. The average number of letters and syllables per adjective was comparable across all four lists. Both French and English versions of the task were developed.
During each trial of the social approval task, a photo of the participant (taken during the self-introductory phase), or of an unfamiliar person of the same gender, replaced a fixation cross at the centre of a black screen. The picture was presented for 1500 ms, followed by a personality trait supposedly attributed to the person depicted in the photo. Each trait was presented under the photo for 3000 ms. Subjects were then asked to rate the desirability of the presented trait on a 3-point scale (1 = Highly desirable; 2 = Moderate; 3 = Not desirable). Subjects provided feedback via a three-button keyboard they were required to hold throughout the duration of the scan. The trial ended with a fixation cross presented for 3000 ms. Thus, the total duration of a trial was 10 seconds. Trials were separated by pseudo-randomized inter-stimulus intervals of 2.7, 3.0 or 3.3 s. For the NoSR condition, the personality trait was replaced by ‘XXXX’ and the subject could press any of the three buttons on the controller. The social approval task consisted of three runs, each consisting of 10 trials per condition, with a total of 60 trials per run. An example of the fMRI task design for a male participant is shown in Figure 1. The total duration of the scanning session lasted ∼50 minutes, including preparation time, delays between runs and T1-weighted image acquisition.
Fig. 1.
FMRI social approval task paradigm. Example for a male participant.
Imaging data acquisition
The social approval task was conducted at the Douglas Institute Brain Imaging Centre, where fMRI data were acquired on a 3T Magnetom MRI Scanner. Stimuli were generated by a laptop running E-PRIME (Psychology Software Tools, Pittsburgh, PA) and projected via an LCD projector and mirror system. The participants’ responses were recorded via a remote control connected to the computer. T2-weighted images with blood oxygen level-dependent contrast were acquired [repetition time (TR) 2000 ms, echo time (TE) 40 ms, flip angle 90°, field of view 256 mm, matrix 64×64], covering the entire brain (30 interleaved slices parallel to the anterior-posterior commissural plane, in-plane resolution 4×4 mm thickness). Three functional runs of 365 volumes each were acquired. Following the functional session, a high-resolution T1-weighted anatomical volume was acquired using a gradient echo pulse sequence (TR 18 ms, TE 10 ms, flip angle 30°, voxel size 1×1×1 mm3).
After completion of the fMRI sequence, participants were required to fill out one last questionnaire, pertaining to their subjective emotional thoughts when presented with each of the traits specifically attributed to them in the scanner. The questionnaire included a seven-point rating scale (1 = very angry, 4 = indifferent, 7 = very happy) for each of the 60 words that represented the results of their personality evaluation. Afterwards, participants were fully debriefed and told about the necessity of the deception approach taken to assess social reward processing.
Statistical analysis for behavioural measures
Demographic and clinical data were compared using two-tailed t tests for independent samples, with the exception of the sex variable, for which a χ2 test was used. Comparisons between patients and controls on all questionnaires from the introductory session of the study were performed using two-tailed t-tests for independent samples. A mixed-design ANOVA was conducted to analyze scores from the post-scan questionnaire, with group as the between subjects variable and reward level (H/LSR) as the within subjects variable. Linear regression analyses were conducted between subjective HSR ratings and other behavioural/neural measures of interest using Pearson R correlation coefficients and significance testing for difference of slopes.
fMRI statistical analysis
FMRI data were analyzed using SPM8 (Wellcome Department of Imaging Neuroscience, UK). All functional volumes from each run were realigned to the first volume to correct for inter-scan movement. To assess the degree of head movement throughout the task, realignment parameters for each individual were visually inspected for the full duration of the scan. Movement parameters were ≤2 mm for translations and ≤ 2° for rotations in x-, y- and z-axes for all participants, with the exception of one patient, whom we excluded from further analyses. Next, realigned volumes were spatially normalized to the Montreal Neurological Institute (MNI) space (normalized voxel size: 2×2×2 mm) and smoothed with an 8-mm full-width half-maximum Gaussian kernel (Friston et al., 2002). Low-frequency temporal drifts were removed by applying a high-pass filter. Data were analyzed by the general linear model (GLM), in which individual events were modelled by a canonical Haemodynamic Response Function.
Our fMRI analysis modelled six events of interest: (i) Self[HSR]; (ii) Self[LSR]; (iii) Self[NoSR]; (iv) Other[HSR]; (v) Other[LSR]; (vi) Other[NoSR]. Ten contrasts of interest were calculated. However, only three of these contrasts are reported and discussed in this paper. First, the effect of receiving positive feedback from others (i.e. social approval) on brain activity was explored using the contrast ‘Self[HSR] vs Other[HSR]’. We then compared results with an opposing contrast: ‘Self[LSR] vs Other[LSR]’. We also explored the neural correlates of receiving a general feedback from others (i.e. irrespective of whether it is positive or negative): ‘Self[HSR+LSR] vs Other[HSR+LSR]’.
The fMRI analysis included six regressors modelling motion-related variance as covariates. One sample t-tests and two sample t-tests were conducted to obtain mean activation maps for each subject and to compare groups for each contrast of interest. A Monte Carlo simulation approach was applied to correct for multiple comparisons, using a software derived by Slotnick and colleagues (Slotnick et al., 2003; Slotnick and Schacter, 2004). A cluster extent threshold was determined using the following input parameters: matrix 64 × 64, slices 30, original voxel dimension 4 × 4 × 4, resampled voxel resolution 2 × 2 × 2, smoothing 8 mm, P-corrected = 0.01, P-voxel = 0.001. After 10 000 iterations, an extent threshold of 80 contiguous voxels was determined. Ultimately, this procedure avoids a false-positive rate above 1% due to multiple testing and provides a method of thresholding that is more sensitive to activation (specifically for low-intensity, spatially extended activation patterns), compared with measures of multiple contiguous activated voxels (Forman et al., 1995; Petersson et al., 1999; Nichols, 2012; Hupé, 2015). For exploratory purposes, the Monte Carlo simulation approach was also run with similar parameters for P-voxel = 0.005, yielding an output of 120 contiguous voxels. Significantly activated clusters were anatomically identified using the Automated Anatomical Labelling software extension within SPM8 (Tzourio-Mazoyer et al., 2002), using an extended local maxima radius of 10 mm. To display fMRI brain activation patterns, resultant statistical maps were overlaid on a T1-weighted Montreal Neurological Institute template using MRIcron (http://www.sph.sc.edu/comd/rorden/mricron/). Average β values for H/LSR-Self and Other conditions were extracted to examine signal change between patients and controls for several regions of interest (ROI), which were defined according to the results of our conjunction analysis (see ‘ROI-specific activation patterns’ in the Results section). The β values across selected ROIs were analyzed with mixed-design ANOVA, with group as a between-subjects factor, and the conditions of interest as a within-subjects factor.
Results
Demographics and behavioural group comparisons
Demographic and clinical data analyses confirm that patients and controls were comparable in terms of age, sex ratio and performance I.Q. (Table 1). However, our groups differed in years of education obtained and verbal I.Q., although I.Q. scores were still well within the average range for patients. Although our patient group scored higher on measures of depression and anxiety compared with controls, as assessed by the CDS and HAS, respectively, these scores were still within the acceptable range and did not reflect significant affective symptomatology. Further information on behavioural data and statistical test results for questionnaires completed during the first session of the study can be found in Table 2. Verbal discussion with participants during the debriefing session after fMRI task acquisition encouraged us that the chosen paradigm effectively convinced both patients and controls that their personality had truly been evaluated.
Table 1.
Demographic and clinical data
| Group; mean (SD) |
Statistical test | |||
|---|---|---|---|---|
| Characteristic | Schizophrenia (n = 15) | Control(n = 15) | P value | |
| Age, year | 33.1 (7.7) | 35.2 (9.8) | T28 = 0.66 | 0.51 |
| Sex, male:female | 11:4 | 9:6 | χ21 = 0.15 | 0.7 |
| Verbal I.Q. | 99.4 (14.0) | 111.3 (10.7) | T28 = 2.623 | 0.014 |
| Performance I.Q. | 103 (15.0) | 105.6 (13.7) | T28 = 0.495 | 0.625 |
| Years of education | 12.5 (2.4) | 15.4 (2.3) | T28 = 3.37 | 0.002 |
| HAS | 5.6 (6.3) | 1.5 (1.8) | T28 = 2.46 | 0.02 |
| CDS | 1.1 (1.6) | 0.1 (0.3) | T28 = 2.55 | 0.02 |
| Language, English:Frencha | 7:8 | 11:4 | χ21 = 1.25 | 0.26 |
| SAPS total score | 11.9 (10.6) | |||
| SANS total score | 19.7 (8.2) | |||
| Age of onset (year) | 21.4 (7.0) | |||
| Number of hospitalizations | 2.5 (2.7) | |||
Individuals with schizophrenia differ from controls in years of education, verbal I.Q., and scores on the HAS and CDS (P < 0.05). No differences were found between age, sex ratio, performance I.Q. and distribution of English and French versions of the task.
aLanguage of preference for each participant defined the language used when conducting clinical/behavioural assessments, and which version of the task was administered in the scanner.
Table 2.
Behavioural measures
| Group; mean (SD) |
Statistical test | |||
|---|---|---|---|---|
| Questionnaire | Schizophrenia (n = 15) | Control (n = 15) | P value | |
| EQ | 35.7 (12.3) | 46.7 (11.0) | T28 = 2.57 | 0.01* |
| PAS | 30.0 (2.9) | 30.1 (3.7) | T28 = 0.11 | 0.91 |
| SAS | 12.8 (7.6) | 8.5 (6.2) | T28 = 1.68 | 0.10 |
| SDS | 17.6 (6.3) | 20.5 (6.5) | T28 = 1.22 | 0.23 |
| SERS | 195.9 (38.6) | 221.6 (25.1) | T28 = 2.16 | 0.04* |
| TEPS | ||||
| TEPSA | 41.2 (8.7) | 45.7 (6.1) | T28 = 1.62 | 0.11 |
| TEPSC | 36.1 (7.0) | 38.4 (5.4) | T28 = 0.99 | 0.33 |
| IRI | ||||
| Perspective-taking | 22.5 (4.9) | 28.6 (5.3) | T28 = 3.23 | 0.003* |
| Fantasy | 21.7 (6.8) | 21.5 (5.9) | T28 = 0.09 | 0.93 |
| Empathic Concern | 24.5 (5.4) | 28.7 (4.0) | T28 = 2.42 | 0.02* |
| Personal Distress | 18.9 (6.3) | 16.1 (5.3) | T28 = 1.32 | 0.20 |
Abbreviations: EQ, empathy quotient; PAS, Physical Anhedonia Scale; SAS, Social Anhedonia Scale; SDS, Social Desirability Scale; SERS, Self-Esteem Rating Scale; TEPS, Temporal Experience of Pleasure Scale (A, Anticipatory; C, Consummatory); IRI, Interpersonal Reactivity Index.
aSignificant differences found between individuals with schizophrenia and controls (P < 0.05).
Subjective ratings of HSR and LSR traits
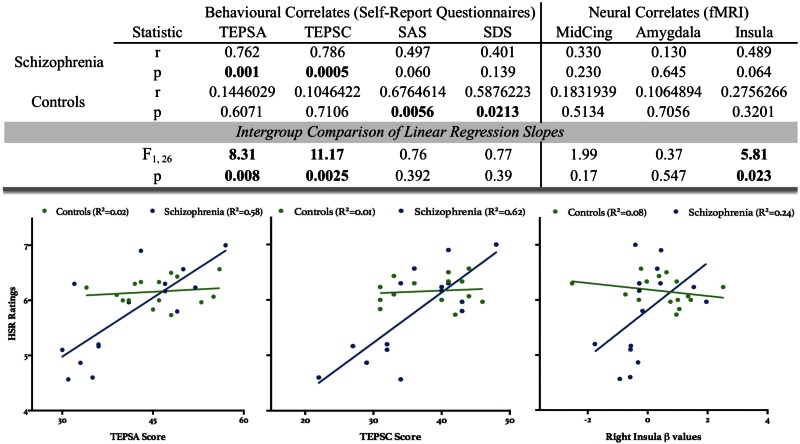
No differences were found between groups with respect to subjective ratings of desirability for HSR and LSR traits in the scanner and reaction times (Supplementary Figure S1). Emotional appraisal of traits from the post-scan questionnaire revealed a significant main effect of reward (Mixed-design ANOVA, F1,28 = 269.3, P < 0.0001), and group × reward interaction (F1,28 = 13.77, P < 0.001). The main effect of reward confirms that subjective feelings of reward were consistent with previously established reward values of the chosen traits, such that traits from the HSR condition elicited more positive affect, and negative traits evoked less favourable subjective reactions (e.g., anger). Post hoc tests of the group × reward interaction, using Tukey’s multiple comparisons, revealed that patients and controls significantly differed in their subjective assessment of LSR words (P = 0.019), where patients exhibited less negative affect when rating LSR words compared with controls (Figure 2). No main effect of language (English or French) was found with respect to subjective ratings of the H/LSR traits, suggesting that the results obtained could not be otherwise explained by the language used during task administration (Supplementary Table S1). Furthermore, subjective ratings of HSR traits from the post-scan questionnaire revealed significantly different correlation patterns with the TEPS, for both the anticipatory (A) and consummatory (C) facets of the scale, where patients’ responses on the TEPS had a significantly higher positive correlation with their ratings on HSR traits, compared with controls. Slope comparisons between ratings on the post-scan questionnaire and other relevant behavioural measures (i.e. SAS and SDS) were not significantly different between groups. Please see Figure 5 for all statistical results.
Fig. 2.
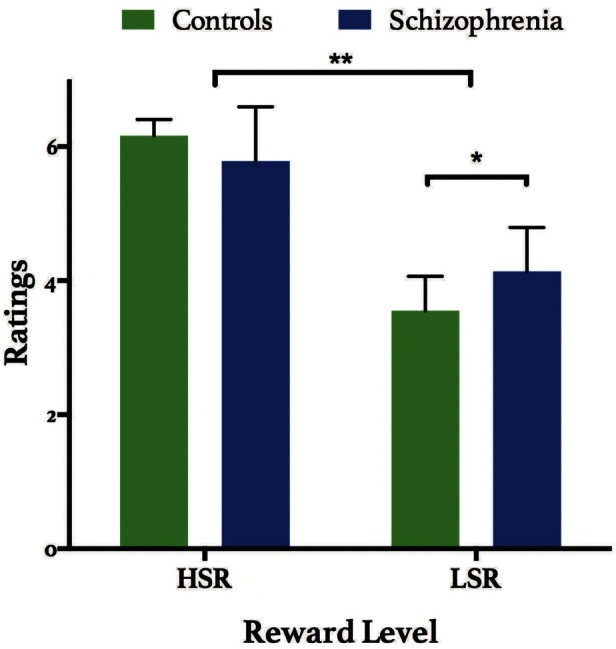
Post-scan questionnaire. Ratings on a seven-point scale of HSR and LSR traits from the ‘self’ condition after the scanning session, ranging from 1 = very angry, 4 = indifferent, 7 = very happy. A main effect of reward was found (Mixed-design ANOVA, F1,28 = 269.3, **P < 0.0001), and a group × reward interaction (F1,28 = 13.77, P < 0.001). Post hoc analyses revealed that patients rated LSR traits significantly lower than controls (*P = 0.019). Bars represent mean ± SD.
Fig. 5.
Correlations with subjective HSR ratings. Abbreviations. TEPSA/C, Temporal Experience of Pleasure—Anticipatory/Consummatory Subscales; SAS, Social Anhedonia Scale. SDS, Social Desirability Scale; MidCing, middle cingulum; All correlations for listed behavioural and neural measures are compared against subjective HSR ratings from the post-scan questionnaire. Correlations with selected neuroanatomical ROIs from the right hemisphere are based on beta values extracted from the ‘Self[HSR]’ Condition. All significant results are bolded within the top panel. When compared with controls, patients with schizophrenia had significantly different slopes compared with controls when examining Pearson R correlation coefficients between subjective HSR trait ratings and TEPSA/C scores, and β values extracted from the right insula for Self[HSR].
FMRI results: Self[HSR] vs Other[HSR]
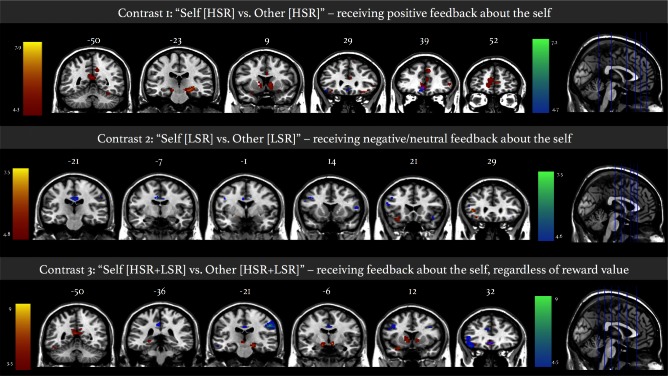
Social reward processing was analyzed through examination of brain activity when presented with specific levels of reward directed towards the self in the scanner. Neural correlates of social approval were first explored by contrasting HSR traits presented in the ‘self’ condition to HSR for the ‘other’ condition. For the described contrast, controls showed activation of various frontal regions bilaterally (i.e. inferior and superior frontal gyri, as well as mPFC), cingulum, several brain regions involved in visual perception (lingual, calcarine and occipital gyri), the precuneus, and temporal regions (bilateral superior temporal pole and fusiform gyrus). Controls also showed activation of specific subcortical regions not found in patients (i.e. thalamus, hippocampus, caudate). Patients showed a similar pattern of activation in frontal regions (i.e. mPFC; superior, middle and inferior frontal gyri; bilateral anterior cingulum). Alternate patterns of activation were found in the patient group as displayed by activity in the cerebellum, rectus, postcentral and supramarginal gyri (Table 3A; Figure 3). A direct comparison between the two groups revealed significantly higher activation within the right parietal lobe compared with patients; specifically, higher activation was found within the middle and posterior cingulum, angular gyrus (AG), and superior and inferior parietal gyri (Table 3A; Figure 4).
Table 3.
Results from fMRI contrasts examining the construct of social approval
|
A. Self [HSR] vs Other [HSR] |
B. Self [LSR] vs Other [LSR] |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MNI Coordinates | MNI Coordinates | ||||||||||||||
| Brain region | BA | H | x | y | z | t-value | Cluster size | Brain region | BA | H | x | y | z | t-value | Cluster size |
| Controls | Controls | ||||||||||||||
| Middle Occipital | 19 | L | −34 | −92 | 8 | 7.94 | 3270 | Inferior frontal, pars triangularis | 47 | L | −40 | 30 | 0 | 7.51 | 2529 |
| Superior Temporal Pole | 47 | L | −30 | 16 | −22 | 7.67 | 1285 | Inferior frontal, pars orbitalis | 47 | L | −30 | 22 | −14 | 6.62 | 1690 |
| Thalamus | L | −10 | −8 | −8 | 7.48 | 1100 | Schizophrenia | ||||||||
| Hippocampus | L | −12 | −10 | −14 | 6.84 | Middle cingulum | 23/24 | B | −8 | −8 | 34 | 7.51 | 1941 | ||
| Caudate | L | −14 | 14 | 2 | 5.22 | Inferior frontal, pars orbitalis | 47 | R | 42 | 22 | −18 | 6.27 | 1707 | ||
| Caudate | R | 10 | 8 | 0 | 5.46 | 1313 | Precentral | 9 | L | −40 | 4 | 36 | 5.79 | 3526 | |
| Superior temporal pole | 38 | R | 36 | 20 | −24 | 7.55 | 1338 | Inferior frontal, pars triangularis | 45 | L | −54 | 20 | 20 | 5.40 | 2529 |
| Inferior frontal, pars orbitalis | 47 | R | 28 | 16 | −22 | 7.22 | Inferior frontal, pars opercularis | 44 | R | 56 | 14 | 10 | 5.30 | 1399 | |
| Superior frontal, pars orbitalis | 11 | R | 24 | 34 | −16 | 5.03 | 997 | Postcentral | 4 | R | 56 | −20 | 36 | 4.88 | 3823 |
| Middle occipital | 19 | R | 44 | −86 | 6 | 7.47 | 2098 | Precuneus | 23 | L | 12 | −54 | 14 | 5.12 | 3528 |
| Precuneus | 31 | R | 10 | −48 | 40 | 7.09 | 3265 | Calcarine | 30 | L | −6 | −56 | 8 | 4.91 | 2258 |
| Precuneus | 31 | L | −14 | −46 | 42 | 5.32 | 3528 | Posterior cingulate | 30 | L | −8 | −54 | 10 | 4.96 | |
| Middle cingulum | 31 | B | −2 | −42 | 38 | 4.31 | 1941 | Angular | 39 | L | −38 | −64 | 32 | 4.89 | 1173 |
| Fusiform | 19 | R | 34 | −66 | −12 | 6.29 | 2518 | Fusiform | 19 | R | 32 | −68 | −10 | 4.61 | 2518 |
| Lingual | 18 | R | 26 | −84 | −2 | 5.08 | 2300 | Controls>Schizophrenia | No significant activation | ||||||
| Inferior occipital | 18 | R | 40 | −84 | −14 | 4.76 | Schizophrenia>Controls | No significant activation | |||||||
| Superior medial frontal | 10 | L | −14 | 56 | 0 | 6.08 | 2992 | C. Self [HSR+LSR] vs other [HSR+LSR] | |||||||
| Superior medial frontal | 9 | R | 8 | 34 | 36 | 6.07 | 2134 | Controls | |||||||
| Anterior cingulum | 24 | B | 6 | 24 | 18 | 5.95 | 1400 | Amygdala | 28 | L | −14 | −4 | −14 | 7.60 | 932 |
| Inferior temporal lobe | 37 | L | −42 | −60 | −8 | 5.86 | 3200 | Parahippocampal | 35 | L | 20 | −24 | −16 | 7.42 | |
| Fusiform | 37 | L | −48 | −60 | −16 | 4.58 | Hippocampus | L | 32 | −24 | −20 | 5.20 | |||
| Inferior frontal, pars triangularis | 45 | R | 52 | 38 | 2 | 5.10 | 2151 | Precuneus | 30 | L | −6 | −54 | 14 | 8.74 | 3528 |
| Schizophrenia | Inferior frontal, pars orbitalis | 47 | L | −26 | 14 | −22 | 7.30 | 1690 | |||||||
| Cerebellum Crus2 | R | 14 | −90 | −38 | 9.05 | 2117 | superior temporal pole | 38 | L | −34 | 20 | −18 | 6.86 | ||
| Cerebellum Crus1 | R | 36 | −86 | −36 | 6.76 | 2648 | Insula | 13 | L | −24 | 22 | −10 | 5.34 | ||
| Postcentral | 3 | R | 64 | −20 | 40 | 7.23 | 3823 | Middle occipital | 19 | R | 42 | −82 | 24 | 6.69 | 2098 |
| Medial orbital frontal | 32 | B | 4 | 32 | −10 | 6.95 | 856 | Superior occipital | 7 | R | 26 | −72 | 42 | 4.54 | 1413 |
| Anterior cingulum | 32 | B | −4 | 36 | −10 | 6.09 | Superior temporal pole | 38 | R | 32 | 20 | −30 | 6.54 | 1338 | |
| Rectus | 25 | L | −4 | 22 | −12 | 4.73 | 852 | Middle Temporal Pole | 11 | R | 30 | 34 | −14 | 4.50 | |
| Middle frontal | 10 | L | −28 | 56 | 24 | 6.62 | 4863 | Insula | 47 | R | 30 | 22 | −18 | 4.76 | 1770 |
| Superior frontal | 10 | L | −20 | 50 | 24 | 5.35 | 3599 | Inferior frontal, pars orbitalis | 11 | R | 30 | 34 | −14 | 4.50 | 1707 |
| Inferior frontal, pars orbitalis | 47 | L | −48 | 28 | −16 | 5.80 | 1690 | Inferior temporal | 19 | L | −44 | −60 | −10 | 6.38 | 3200 |
| Controls > Schizophrenia | Fusiform | 37 | L | −48 | −54 | −22 | 5.91 | 2603 | |||||||
| P < 0.001 | Middle occipital | 19 | L | −32 | −88 | 4 | 6.02 | 3270 | |||||||
| Middle cingulum | 31 | R | 10 | −44 | 38 | 4.85 | 2203 | Lingual | 18 | L | −24 | −92 | −10 | 5.48 | 2095 |
| Posterior cingulum | 31 | R | 18 | −44 | 40 | 3.46 | Anterior cingulum | 32 | B | 0 | 36 | −4 | 5.95 | 1313 | |
| Angular | 40 | R | 46 | −58 | 40 | 4.51 | 1752 | Medial orbital frontal | 10 | B | 8 | 46 | 18 | 5.60 | |
| Superior parietal | 40 | R | 36 | −54 | 56 | 3.97 | 1345 | Superior medial frontal | 6 | R | 6 | 36 | 40 | 5.82 | 2134 |
| Inferior parietal | 40 | R | 40 | −40 | 54 | 3.90 | Middle cingulum | 24 | R | 2 | 4 | 30 | 5.23 | 2203 | |
| P < 0.005 | Middle cingulum | 24 | L | 0 | −12 | 36 | 5.04 | 1941 | |||||||
| Precuneus | 7 | L | −14 | −44 | 58 | 4.29 | 3528 | Schizophrenia | |||||||
| Insula | 13 | R | 30 | 12 | −18 | 4.03 | 1770 | Postcentral | 3 | R | 48 | −20 | 38 | 6.91 | 3823 |
| Superior temporal pole | 47 | R | 34 | 18 | −22 | 3.58 | 1338 | Precentral | 6 | R | 60 | −12 | 40 | 6.77 | 3381 |
| Amygdala | R | 24 | 2 | −12 | 3.44 | 248 | Inferior frontal, pars orbitalis | 47 | L | −46 | 30 | −16 | 5.62 | 1690 | |
| Superior medial frontal | 8 | R | 10 | 34 | 38 | 4.00 | 2134 | Superior temporal pole | 38 | L | −46 | 18 | −12 | 3.58 | 1285 |
| Superior frontal | 6 | R | 22 | 20 | 50 | 3.74 | 4056 | Angular | 39 | L | −40 | −66 | 34 | 5.55 | 1173 |
| Middle frontal | 6 | R | 30 | 24 | 52 | 3.22 | 5104 | Precentral | 6 | L | −44 | 0 | 36 | 5.29 | 3526 |
| Posterior cingulum | 31 | L | −24 | −48 | 24 | 3.70 | 1526 | Middle frontal | 9 | L | −40 | 12 | 34 | 5.00 | 4863 |
| Angular | 39 | L | −30 | −54 | 26 | 3.33 | 1173 | Controls > Schizophrenia | No significant activation | ||||||
| Schizophrenia>Controls | No significant activation | Schizophrenia>Controls | No significant activation | ||||||||||||
Abbreviations: BA, Brodmann’s area; H, hemisphere; L, left; R, right; B, bilateral. x-, y- and z- coordinates of local maxima are reported in MNI space. Significant activations reflect a threshold of P < 0.001 and cluster-extent threshold of 80 voxels. Additional subthreshold clusters have been reported for the Self[HSR] vs Other[HSR] contrast (Panel A) directly comparing controls and patients, with a threshold of P < 0.005, and a resampled cluster size of 120 voxels.
Fig. 3.
fMRI intragroup activation maps. Superposition of activation maps for controls and patients displayed at P < 0.001 with a cluster extent threshold of 80 voxels. Colour bar represents t-scores, where statistical maps are superimposed on the Montreal Neurological Institute template brain. Red-yellow maps correspond to activation in healthy control group. Blue-green maps represent activation in schizophrenia group. Overlapping activation is displayed in pink. Numbers above coronal slices represent y-coordinate. Numbers above axial sections represent x-coordinate. Left side of image corresponds to left side of the brain.
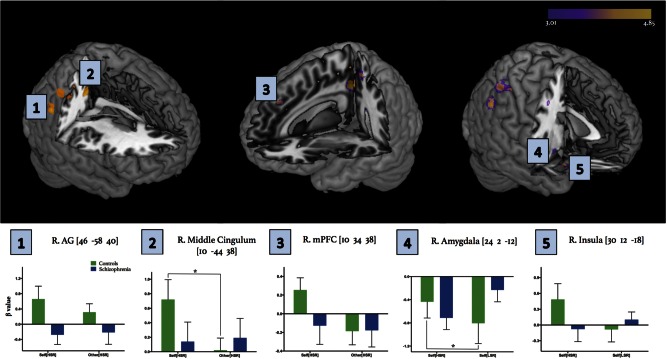
Fig. 4.
Intergroup differences within fMRI activation maps for Self[HSR] vs Other[HSR] and related conditions. Abbreviations. R, right hemisphere; AG, angular gyrus; mPFC, medial prefrontal cortex. Superposition of activation maps directly comparing controls and patients using two thresholds: P < 0.001 and P < 0.005, with a cluster extent threshold of 80 and 120 voxels, respectively. Activations corresponding to the initial conservative P < 0.001 threshold are depicted in yellow, whereas additional activation captured by the more liberal P < 0.005 threshold are depicted in purple. Colour bar represents t-scores, where statistical maps are superimposed on the Montreal Neurological Institute template brain. Selected ROIs are indicated numerically on the activation map panel, with corresponding β values extracted around the peak voxel indicated for each ROI, signifying signal change between patients and controls for separate conditions. Note that β value bar graphs for regions 1-3 compare Self[HSR] and Other[HSR] conditions, whereas regions 4–5 compare Self[HSR] and Self[LSR] conditions due to the latter (i.e. insula and amygdala) having proposed roles in valence/reward processing. Mixed-design ANOVA analyses, with condition as a within subjects factor and group as a between subjects factor, revealed significant interaction effects within the right middle cingulum (F1,28 = 5.067, P = 0.032), amygdala (F1,28 = 2.964, P = 0.0027) and insula (F1,28 = 4.355, P = 0.046). Post hoc analyses revealed a significant difference in β values between conditions for controls within the amygdala and middle cingulum (*P < 0.05), although there was a noteworthy similar trend within the right insula (P = 0.066). Bars represent mean ± SEM.
Due to the noteworthy pattern of limbic lobe activation in controls when examining results at the intragroup level, and evidence suggesting that between-group comparisons are often prone to small effect sizes (Lakens, 2013; Hawco et al., 2015), it was of interest to see if any potential trends would emerge by using a less conservative approach. Thus, a further exploratory analysis of this contrast was conducted using a more liberal threshold of P < 0.005 and cluster extent threshold of 120 voxels, corrected to P < 0.01 at the cluster level (determined with the Monte Carlo simulation approach described earlier). This revealed additional higher activation in controls compared with patients in extended limbic regions of the right hemisphere, including the insula, superior temporal pole, amygdala, as well as additional cortical midline structures including the superior mPFC and posterior cingulum (Table 3A, Figure 4).
ROI-specific activation patterns
To further investigate specific activation patterns for the conditions of interest, average β values were extracted for five ROIs within the right hemisphere, based on our resultant neural group differences: AG, middle cingulum, mPFC, amygdala and anterior insula (Figure 4). To visualize activation patterns across these five selected regions more clearly, β values were extracted for an ROI of 11 voxels (9 in-plane voxels surrounding the peak voxel of the chosen ROI, along with one voxel above and below). Mixed-design ANOVA analyses revealed significant interaction effects within the right middle cingulum (F1,28 = 5.067, P = 0.032), amygdala (F1,28 = 2.964, P = 0.0027), and insula (F1,28 = 4.355, P = 0.046) for resultant β values. Post hoc analyses revealed a significant difference in activation between conditions for controls within the amygdala only (P < 0.05), although there was a noteworthy similar trend within the right insula (P = 0.066). To link neural activation patterns to behavioural responses, subjective ratings of HSR traits from the post-scan questionnaire were correlated with β values representing the Self[HSR] condition across the right middle cingulum, amygdala and anterior insula. Patients had significantly higher positive correlations between subjective ratings of HSR traits from the post-scan questionnaire and activation within the right insula compared with controls (Figure 5). Linear regression analyses between HSR trait ratings and the other aforementioned neuroanatomical regions were not significantly different between groups.
Self[LSR] vs Other[LSR]
A complementary contrast was examined with respect to brain activation when receiving negative feedback about the self, compared with the ‘Other[LSR]’ condition. Only the left inferior frontal gyrus was significantly activated in controls. In contrast, patients showed additional activation of the right frontal cortex, parietal regions (i.e. precuneus, AG), left calcarine gyrus and right fusiform gyrus. No significant differences in brain activation were found when directly comparing patients and controls (Table 3B; Figure 3).
Self[HSR + LSR] vs Other[HSR+LSR]
Brain regions related to self-processing were examined by comparing brain activation during the self and other conditions, irrespective of reward valence. In healthy controls, medial prefrontal regions were activated bilaterally (i.e. medial orbital and superior medial frontal gyri; anterior cingulum), along with the hippocampus and amygdala subcortically. Controls also showed significant activation of several temporal regions (i.e. superior, middle and inferior temporal gyri, as well as fusiform and parahippocampal gyri), parietal areas (i.e. precuneus and cingulum), and within the occipital lobe (i.e. superior and middle occipital gyri). By comparison, patients with schizophrenia displayed activity of the right pre- and post-central gyri, and within the parietal lobule (i.e. supramarginal and angular gyri). Direct comparison between groups revealed no significant differences (Table 3C; Figure 3).
Discussion
Social cognition is an integral component of interpersonal communication, and dysfunction within neural networks underlying social functioning may be implicated in schizophrenia. This study aimed to elucidate the neural correlates of social reward processing using abstract social reinforcers presented in an fMRI paradigm. The principal objectives were to uncover self-referential processing when receiving feedback in the scanner, while also manipulating the reward level of the presented traits. The resultant feedback that each participant received served as a basis for the reputation they embodied, and thus introduced a salient abstract reward. Our neuroimaging results suggest that patients with schizophrenia utilize different brain regions when assessing social reward specific to the self (i.e. social approval), compared with the healthy control group.
We initially hypothesized that individuals with schizophrenia would have an altered subjective response when viewing positive personality traits from their alleged evaluation. Intriguingly, patients rated words from the LSR condition as slightly less unpleasant than what was seen in healthy controls. This suggests that individuals with schizophrenia still retain the ability to differentiate between high and low valence levels of an abstract reward, but have reduced aversion to LSR. Although neurobiological results relating to LSR did not yield significant differences between groups, we were able to uncover several other links between both neural and subjective tendencies relating to the pattern of responses on the post-scan questionnaire. Subjective HSR ratings were found to be quite variable among our patient group, and were positively correlated with patients’ self-reported experience of pleasure, which was not found in controls. It is probable that patients have similar cognitive processes underlying various domains of abstract reward processing, and improving perception of social reward may have beneficial translational effects for other relevant cognitive domains.
One of the primary aims of the study was to decipher some of the mechanisms underlying social approval and abstract reward processing in schizophrenia. Thus, brain activation was examined while viewing self-related HSR traits specifically [i.e. ‘Self(HSR) vs Other(HSR)’]. Our most robust finding was significantly higher activation of several contiguous regions within the parietal lobe and along the posterior cortical midline in healthy controls compared with individuals with schizophrenia. Although these regions were not initially hypothesized to contribute to social reward processing, the parietal lobe has recently gained increased interest in the context of abnormal psychology (Sowden and Shah, 2014). For instance, cognitive processes underlying self-referential processing and the self-other dichotomy may have their neural substrates within the functional network connecting the right temporoparietal junction and mPFC (Spengler et al., 2010). Additionally, differential activation patterns between groups within the posterior portion of the middle cingulum in response to positive feedback deserves recognition, due to this cortical midline structure’s known role in self-referential processing in the healthy population (Northoff and Bermpohl, 2004; Johnson et al., 2002), which evidently, does not function in a comparable manner in our patient group. These abnormalities in posterior midline structures and parietal network connectivity in the context of self-referential processing is consistent with several other recent studies (Murray et al., 2012; Guo et al., 2014; Murray et al., 2015).
It should be noted that the selected threshold may have been too stringent for our modest sample size; thus no direct group differences were found for other subcortical structures of interest. We followed up with a secondary analysis of the ‘Self[HSR] vs Other[HSR]’ contrast using a more liberal threshold of P < 0.005 to explore clusters that may have fallen short of reaching significance in our initial analysis. By lowering the threshold slightly, we found significantly higher activation in controls in regions falling in line with our initial hypotheses (i.e. mPFC), as well as regions strongly implicated in valence processing (i.e. amygdala, insula). We incorporated these new ROIs with our initial findings of significant differential activation in the right parietal lobe (i.e. middle cingulum, AG) to survey activation patterns across conditions of interest (particularly, conditions underlying ‘self’ and ‘HSR’ processing). In this manner, we were able to disentangle the directionality of activation patterns between groups within the aforementioned regions, revealing particularly striking results within the right amygdala and anterior insula. Specifically, the right anterior insula emerged as a potential biological link between neural response patterns and subjective experience of receiving positive feedback, such that lower activation within this region corresponded to lower HSR ratings. Notably, this relationship was specific to the patient group, suggesting that higher right insular activation, approximating levels seen in controls, may be related to a more positive experience of social reward. Previous reports have claimed there is a prominent reduction in connectivity between the amygdala and insula, as well as global abnormalities in the insula-anterior cingulate network for the processing of salient stimuli (Gradin et al., 2013; Mukherjee et al., 2014). A recent report has also highlighted the right anterior insula as a significant predictor of cognition (Sheffield et al., 2015), providing support to our findings of a potential association between this region and social reward processing.
Convincing participants their personality would be evaluated targeted a highly socially relevant construct of reputation. Being mindful of one’s own reputation involves constructing a representation of how others view us, and requires meta-knowledge of the self (Ochsner et al., 2005). As inherently social beings, individuals are acutely aware of the social environment and the relative reputation each person holds within their peer group. At the neural level, our patient group did not show clear discrimination of the self-other dichotomy in regions that have been implicated in self-processing, such as cortical midline structures and associated limbic regions. However, our behavioural results indicate that subjective response to positive feedback can be linked to other domains of pleasure and abstract reward in patients, suggesting that a treatment approach targeting these constructs more globally may yield effective outcomes for patients with schizophrenia.
It is acknowledged that the presented results are limited by a small sample size. This restricted us from uncovering which regions were more highly activated in controls compared with patients, and vice versa, when using a conventional stringent threshold for significant activation, although we uncovered additional findings upon application of a more liberal threshold. Thus, our findings are exploratory in nature but still hold value for directing future work in social cognitive mechanisms underlying mental health disorders such as schizophrenia. Additionally, patients reached a significantly lower level of educational achievement compared with controls but it is unlikely that differences in intelligence levels contributed to our results, as I.Q. scores fell within the average range in our patient group. Finally, both Anglophone and Francophone participants were included in this study. However, English and French versions of each clinical and behavioural measure included in our methods have previously been shown to be equivalent in terms of validity and reliability. The words chosen for HSR and LSR conditions were also carefully selected for each version of the fMRI task to ensure consistency in presented reward levels; thus, it is improbable that language differences played a role in explaining our findings.
Conclusion
The presented findings suggest differential brain activation in patients with schizophrenia when receiving an abstract social reward, compared with healthy controls. Individuals with schizophrenia seem to exhibit differential involvement of midline cortical structures and parietal regions relative to controls, which has possible links with emotional response to social rewards. Our results suggest that at the neural level, patients show altered cognitive processing regarding their social reputation, accompanied by subtle differences in their subjective emotional responses to varying levels of reward presented in an evaluative context. Social cognition is not a unitary construct and relies on various neural circuits, of which certain brain structures may be inconsistently activated in schizophrenia. With increased knowledge of the sociocognitive impairments characterizing schizophrenia, future treatment plans may benefit from incorporating a more socially directed component into applied behavioural therapies, with the ultimate aim of facilitating prosocial behaviours among patients.
Supplementary Material
Acknowledgements
The authors thank the Lepage Lab for their contribution to recruitment of participants for the study and patient evaluations, and Michael Bodnar, Synthia Guimond and Colin Hawco for providing additional resources for fMRI analysis.
Funding
This work was supported by the Quebec Bio-imaging Network (QBIN)/Fonds de Recherche en Santé du Québec (FRSQ) (grant 11/59).
Supplementary data
Supplementary data are available at SCAN online.
Conflict of interest. None declared.
References
- Addington D., Addington J., Maticka-Tyndale E. (1994). Specificity of the Calgary Depression Scale for schizophrenia. Schizophrenia Research, 11, 239–44. [DOI] [PubMed] [Google Scholar]
- Anderson N.H. (1968). Likableness ratings of 555 personality-trait words. Journal of Personality and Social Psychology, 9, 272–9. [DOI] [PubMed] [Google Scholar]
- Andreasen N.C. (1984a). Scale for the Assessment of Positive Symptoms (SAPS). Iowa City: University of Iowa. [Google Scholar]
- Andreasen N.C. (1984b). Scale for the Assessment of Negative Symptoms (SANS). Iowa City: University of Iowa. [Google Scholar]
- Barbour T., Murphy E., Pruitt P., et al. (2010). Reduced intra-amygdala activity to positively valenced faces in adolescent schizophrenia offspring. Schizophrenia Research, 123, 126–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch D.M. (2008). Emotion, motivation, and reward processing in schizophrenia spectrum disorders: what we know and where we need to go. Schizophrenia Bulletin, 34, 816–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch D.M., Dowd E.C. (2010). Goal representations and motivational drive in schizophrenia: the role of prefrontal-striatal interactions. Schizophrenia Bulletin, 36, 919–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron-Cohen S., Wheelwright S. (2004). The empathy quotient: an investigation of adults with Asperger syndrome or high functioning autism, and normal sex differences. Journal of Autism and Developmental Disorders, 34, 163–75. [DOI] [PubMed] [Google Scholar]
- Bateson M., Nettle D., Roberts G. (2006). Cues of being watched enhance cooperation in a real-world setting. Biology Letters 2, 412–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellack A.S., Morrison R.L., Wixted J.T., Mueser K.T. (1990). An analysis of social competence in schizophrenia. The British Journal of Psychiatry, 156, 809–18. [DOI] [PubMed] [Google Scholar]
- Benabou R., Tirole J. (2006). Incentives and prosocial behavior. American Economic Review, 96, 1652–78. [Google Scholar]
- Boksem M.A.S., Ruys K.I., Aarts H. (2011). Facing disapproval: performance monitoring in a social context. Social Neuroscience, 6, 360–8. [DOI] [PubMed] [Google Scholar]
- Brekke J., Kay D.D., Lee K.S., Green M.F. (2005). Biosocial pathways to functional outcome in schizophrenia. Schizophrenia Research, 80, 213–25. [DOI] [PubMed] [Google Scholar]
- Chapman L.J., Chapman J.P., Raulin M.L. (1976). Scales for physical and social anhedonia. Journal of Abnormal Psychology, 85, 374–82. [DOI] [PubMed] [Google Scholar]
- Cohen A.S., Najolia G.M., Brown L.A., Minor K.S. (2011). The state-trait disjunction of anhedonia in schizophrenia: potential affective, cognitive and social-based mechanisms. Clinical Psychology Review, 31, 440–8. [DOI] [PubMed] [Google Scholar]
- Combs D.R., Penn D.L., Wicher M., Waldheter E. (2007). The Ambiguous Intentions Hostility Questionnaire (AIHQ): a new measure for evaluating hostile social-cognitive biases in paranoia. Cognitive Neuropsychiatry, 12, 128–43. [DOI] [PubMed] [Google Scholar]
- Crowne D.P., Marlowe D. (1960) A new scale of social desirability independent of psychopathology. Journal of Consulting Psychology, 24, 349–54. [DOI] [PubMed] [Google Scholar]
- Couture S.M., Penn D.L., Roberts D.L. (2006). The functional significance of social cognition in schizophrenia: a review. Schizophrenia Bulletin, 32, S44–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M.H. (1980) A multidimensional approach to individual differences in empathy. JSAS Catalog of Selected Documents in Psychology, 10, 85. [Google Scholar]
- Diekhof E.K., Falkai P., Gruber O. (2008) Functional neuroimaging of reward processing and decision-making: a review of aberrant motivational and affective processing in addiction and mood disorders. Brain Research Reviews, 59, 164–84. [DOI] [PubMed] [Google Scholar]
- First M.B., Gibbon M., Spitzer R.L., Williams J.B.W., Benjamin L.S. (1996). User’s Guide for the Structured Clinical Interview for DSM-IV Axis I Disorders. New York: New York State Psychiatric Institute. [Google Scholar]
- First M.B., Gibbon M., Spitzer R.L., Williams J.B.W., Benjamin L.S. (1997). Structured Clinical Interview for DSM-IV Axis II Personality Disorders (SCID-II). Washington, DC: American Psychiatric Press, Inc. [Google Scholar]
- Forman S.D., Cohen J.D., Fitzgerald M., et al. (1995). Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magnetic Resonance in Medicine, 33, 636–47. [DOI] [PubMed] [Google Scholar]
- Friston K.J., Glaser D.E., Henson R.N., et al. (2002). Classical and Bayesian inference in neuroimaging: applications. Neuroimage, 16, 484–512. [DOI] [PubMed] [Google Scholar]
- Galderisi S., Rossi A., Rocca P., et al. (2014). The influence of illness-related variables, personal resources and context-related factors on real-life functioning of people with schizophrenia. World Psychiatry, 13, 275–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gard D.E., Fisher M., Garrett C., Genevsky A., Vinogradov S. (2009). Motivation and its relationship to neurocognition, social cognition, and functional outcome in schizophrenia. Schizophrenia Research, 115, 74–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gard D.E., Gard M.G., Kring A.M., John O.P. (2006). Anticipatory and consummatory components of the experience of pleasure: a scale development study. Journal of Research in Personality, 40, 1086–102. [Google Scholar]
- Gard D.E., Kring A.M., Gard M.G., Horan W.P., Green M.F. (2007). Anhedonia in schizophrenia: distinctions between anticipatory and consummatory pleasure. Schizophrenia Research, 93, 253–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold J.M., Waltz J.A., Prentice K.J., Morris S.E., Heerey E.A. (2008).Reward processing in schizophrenia: a deficit in the representation of value. Schizophrenia Bulletin, 34, 835–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradin V.B., Waiter G., O’Connor A., et al. (2013). Salience network-midbrain disconnectivity and blunted reward signals in schizophrenia. Psychiatry Research: Neuroimaging, 211, 104–11. [DOI] [PubMed] [Google Scholar]
- Guo S., Kendrick K.M., Yu R., Wang H.L., Feng J. (2014). Key functional circuitry altered in schizophrenia involves parietal regions associated with sense of self. Human Brain Mapping, 35, 123–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M. (1959). The assessment of anxiety states by rating. British Journal of Medical Psychology, 32, 50–5. [DOI] [PubMed] [Google Scholar]
- Harvey P.O., Fossati P., Lepage M. (2007). Modulation of memory formation by stimulus content: specific role of the medial prefrontal cortex in the successful encoding of social pictures. Journal of Cognitive Neuroscience, 19, 351–62. [DOI] [PubMed] [Google Scholar]
- Hawco C., Buchy L., Bodnar M., et al. (2015). Source retrieval is not properly differentiated from object retrieval in early schizophrenia: an fMRI study using virtual reality. Neuroimage: Clinical 7, 336–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hays J.R., Reas D.L., Shaw J.B. (2002). Concurrent validity of the Wechsler abbreviated scale of intelligence and the Kaufman brief intelligence test among psychiatric inpatients. Psychological Reports, 90, 355–9. [DOI] [PubMed] [Google Scholar]
- Horan W.P., Kring A.M., Blanchard J.J. (2006). Anhedonia in schizophrenia: a review of assessment strategies. Schizophrenia Bulletin, 32, 259–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hupé J.M. (2015). Statistical inferences under the Null hypothesis: common mistakes and pitfalls in neuroimaging studies. Frontiers in Neuroscience, 9, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izuma K., Saito D.N., Sadato N. (2008). Processing of social and monetary rewards in the human striatum. Neuron, 58, 284–94. [DOI] [PubMed] [Google Scholar]
- Johnson S.C., Baxter L.C., Wilder L.S., et al. (2002). Neural correlates of self-reflection. Brain 125, 1808–14. [DOI] [PubMed] [Google Scholar]
- Koch K., Schachtzabel C., Wagner G., et al. (2010). Altered activation in association with reward-related trial-and-error learning in patients with schizophrenia. Neuroimage, 223–32. [DOI] [PubMed] [Google Scholar]
- Kurzban R., Aktipis C.A. (2007). Modularity and the social mind: are psychologists too selfish? Personality and Social Psychology Review, 11, 131–49. [DOI] [PubMed] [Google Scholar]
- Lakens D. (2013). Calculating and reporting effect sizes to facilitate cumulative science: a practical primer for t-tests and ANOVAs. Frontiers in Psychology: Cognition, 4, 863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leary M.R. (2007). Motivational and emotional aspects of the self. Annual Review of Psychology,58, 317–44. [DOI] [PubMed] [Google Scholar]
- Million T., Davis R., Million C. (1997). Millon Clinical Multiaxial Inventory III: Manual. Minneapolis: National Computer Systems. [Google Scholar]
- Moran J.M., Macrae C.N., Heatherton T.F., Wyland C.L., Kelley W.M. (2006). Neuroanatomical evidence for distinct cognitive and affective components of self. Journal of Cognitive Neuroscience, 18, 1586–94. [DOI] [PubMed] [Google Scholar]
- Mukherjee P., Whalley H.C., McKirdy J.W., et al. (2014). Altered amygdala connectivity within the social brain in schizophrenia. Schizophrenia Bulletin, 40, 152–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray G.K., Corlett P.R., Clark L., et al. (2008). Substantia nigra/ventral tegmental reward prediction error disruption in psychosis. Molecular Psychiatry, 13, 267–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray R.J., Debbané M., Fox P.T., Bzdok D., Eickhoff S.B. (2015). Functional connectivity mapping of regions associated with self- and other-processing. Human Brain Mapping, 36, 1304–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray R.J., Schaer M., Debbane M. (2012). Degrees of separation: a quantitative neuroimaging meta-analysis investigating self-specificity and shared neural activation between self- and other-reflection . Neuroscience and Biobehavioral Reviews, 36, 1043.–. [DOI] [PubMed] [Google Scholar]
- Nichols T.E. (2012). Multiple testing corrections, nonparametric methods, and random field theory. Neuroimage, 62, 811–5. [DOI] [PubMed] [Google Scholar]
- Northoff G., Bermpohl F. (2004). Cortical midline structures and the self. Trends in Cognitive Science, 8, 102–7. [DOI] [PubMed] [Google Scholar]
- Northoff G., Heinzel A., Bermpohl F., Dobrowolny H., Panksepp J. (2006). Self-referential processing in our brain - a meta-analysis of imaging studies on the self. Neuroimage, 31, 440–57. [DOI] [PubMed] [Google Scholar]
- Nugent W.R., Thomas J.W. (1993). Validation of a clinical measure of self-esteem. Research on Social Work Practice, 3, 191–207. [Google Scholar]
- Ochsner K.N., Beer J.S., Robertson E.R., et al. (2005). The neural correlates of direct and reflected self-knowledge. Neuroimage, 28, 797–814. [DOI] [PubMed] [Google Scholar]
- Penn D.L., Corrigan P.W., Bentall R.P., Racenstein J.M., Newman L. (1997). Social cognition in schizophrenia. Psychological Bulletin, 121, 114–32. [DOI] [PubMed] [Google Scholar]
- Petersson K.M., Nichols T.E., Poline J.B., Holmes A.P. (1999). Statistical limitations in functional neuroimaging. II. Signal detection and statistical inference. Philosophical Transactions of the Royal Society B: Biological Sciences, 354, 1261–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkham A.E., Penn D.L., Perkins D.O., Lieberman J. (2003). Implications for the neural basis of social cognition for the study of schizophrenia. The American Journal of Psychiatry, 160, 815–24. [DOI] [PubMed] [Google Scholar]
- Sheffield J.M., Repovs G., Harms M.P., et al. (2015). Fronto-parietal and cingulo-opercular network integrity and cognition in health and schizophrenia. Neuropsychologia, 73, 82–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon J.J., Biller A., Walther S., et al. (2010). Neural correlates of reward processing in schizophrenia – Relationship to apathy and depression. Schizophrenia Research, 118, 154–61. [DOI] [PubMed] [Google Scholar]
- Slotnick S.D., Moo L.R., Segal J.B., Hart J., Jr (2003). Distinct prefrontal cortex activity associated with item memory and source memory for visual shapes. Cognitive Brain Research, 17, 75–82. [DOI] [PubMed] [Google Scholar]
- Slotnick S.D., Schacter D.L. (2004). A sensory signature that distinguishes true from false memories. Nature Neuroscience, 7, 664–72. [DOI] [PubMed] [Google Scholar]
- Sowden S., Shah P. (2014). Self-other control: a candidate mechanism for social cognitive function. Frontiers in Human Neuroscience, 8, 789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spengler S., von Cramon D.Y., Brass M. (2010). Resisting motor mimicry: control of imitation involves processes central to social cognition in patients with frontal and temporo-parietal lesions. Social Neuroscience, 5, 401–16. [DOI] [PubMed] [Google Scholar]
- Strauss G.P., Waltz J.A., Gold J.M. (2014). A Review of Reward Processing and Motivational Impairment in Schizophrenia. Schizophrenia Bulletin, 40, S107–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N., Landeau B., Papathanassiou D., et al. (2002). Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage, 15, 273–89. [DOI] [PubMed] [Google Scholar]
- van der Meer L., Costafreda S., Aleman A., David A.S. (2010). Self-reflection and the brain: a theoretical review and meta-analysis of neuroimaging studies with implications for schizophrenia. Neuroscience and Biobehavioral Reviews, 34, 935–46. [DOI] [PubMed] [Google Scholar]
- Waltz J.A., Schweitzer J.B., Gold J.M., et al. (2009). Patients with schizophrenia have a reduced neural response to both unpredictable and predictable primary reinforcers. Neuropsychopharmacology, 34, 1567–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waltz J.A., Schweitzer J.B., Ross T.J., et al. (2010). Abnormal responses to monetary outcomes in cortex, but not in the basal ganglia, in schizophrenia. Neuropsychopharmacology, 35, 2427–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.