Abstract
Study Design
Establishment of immortalized cell lines derived from rat intervertebral disc cells by Rock inhibitor, Y-27632.
Objective
To determine whether rat nucleus pulposus (NP) and annulus fibrosus (AF) cells could be immortalized, retain their phenotype and used as cell lines for in vitro cell biology.
Summary of Background Data
Intervertebral disc degeneration is a major factor for most low-back pain. However, the mechanism of the disease is not well understood by the limitation to obtain sufficient amounts of primary disc cells. Therefore, the establishment of disc cell lines will help in vitro molecular signaling studies to understand the mechanism of degenerative disc disease.
Methods
Cells were isolated from the NP and AF tissues of lumbar discs of adult Sprague Dawley rat. Tissues were digested and cultured with DMEM/Ham's F-12 (1:1) and 20% FBS and antibiotics. From day 3, cells were grown in the presence of 10 μM Y-27632, a well-characterized inhibitor of the Rho-associated kinase (ROCK), and subcultured by trypsinization, passaging them 1:3 onto 100 mm culture dishes. Morphologic and genetic analyses were performed on the different passaged cells.
Results
ROCK inhibitor successfully immortalized rat NP and AF cells. They passaged for over 50 generations with sustained expression levels of several NP and AF cell markers. Additionally, they retained phenotypic features similar to the primary parental NP and AF cells when the cells were challenged with different cytokines and growth factors.
Conclusions
We established immortalized rat NP and AF cell lines using a method of treating cells with ROCK inhibitor Y-27632 and demonstrated that these immortalized cells retain the properties of primary cells and could serve as useful tools for in vitro signaling studies or drug screening studies to develop novel therapeutic strategies.
Keywords: Intervertebral disc, nucleus pulposus, annulus fibrosus, cell line, ROCK inhibitor
INTRODUCTION
The intervertebral disc provides flexibility to the spine, allowing for movements and transmission of mechanical loads. These include the central gel-like nucleus pulposus (NP), which is peripherally contained by the annulus fibrosus (AF), and the cartilaginous endplates (CEP) and growth plate that anchor the discs to the adjacent vertebral bodies [1-3]. NP is highly hydrated and concentrated with proteoglycans. During IVD degeneration, the synthesis of extracellular matrix proteins changes in NP, leading to the loose of osmotic properties. The AF is composed of inner and outer parts with different levels of type I and type II collagen expression. The changes in collagen expression within the inner part of AF can affect mechanical behavior of IVD [4]. Disc degeneration begins with changes in the cellular function within the substructures of the disc by the loss of water-binding proteoglycans through increased degradation, causing an overall shift toward a more fibrotic matrix that reduces disc height and alters mechanical loading [5]. Since low back pain is a major public health problem associated with disc degeneration and causes suffering and distress to patients, understanding of the molecular mechanism of this disease is important for the treatment of disc degeneration [6]. However, the studies are hampered by the restriction of the availability of intact disc cells derived from human or rodent disc tissues that would enable more comprehensive analyses and primary cultures of disc cells reduced cell proliferation and differentiation properties and lost original phenotype during cell culture expansion. The goal of this study is to establish disc cell lines which allow in vitro signaling studies and drug screening. In previous reports, to establish disc cell lines, human NP cells were immortalized by transfection with HPV-16 E6/E7 gene [7] or hTERT gene [8]. In addition, Sakai group introduced original-defective simian virus 40 (SV40) early gene into human NP cells by a recombinant SV40 adenovirus vector (AdSV40) [9]. In recent years, genetic mouse models and rats have been used to study the mechanism of disc degeneration. Rodent NP and AF cell lines could be used as complimentary tools for in vivo animal studies and to compare effects of growth factor signaling in both NP and AF cells so in the present studies, we have established immortalized rat NP and AF cell lines.
Although it is possible to immortalize primary cells with the previous methods, it has recently been shown that inhibition of Rho-associated kinase (ROCK) greatly stimulated efficient keratinocyte immortalization [10], and that the ROCK inhibitor, in combination with fibroblast feeder cells, induced normal and tumor epithelial cells from many types of tissues to proliferate indefinitely in vitro [11]. The aim of this study was to investigate whether the ROCK inhibitor can be used to immortalize primary disc cells. Another aim is to characterize the immortalized NP and AF cells by analyzing changes in cell morphology, cell proliferation, and gene expression.
MATERIALS AND METHODS
Cell culture of rat NP and AF cells
Cells were isolated from NP and AF tissues in lumbar discs from adult Sprague Dawley rat weighing between 250-300g. The cells were treated with 0.2% pronase and 0.025% collagenase P (Sigma) overnight for digestion. Rat NP and AF cells were isolated using a method reported by Ellman et al., 2012 [12] and cultured with Dulbecco's modified Eagle's medium (DMEM)/Ham's F-12 (1:1) (Gibco) supplemented with 20% fetal bovine serum (FBS) (Gibco), and 1% (vol/vol) penicillin and streptomycin in a 37°C, 5% CO2 atmosphere. From day 3 onward, cells were grown in the presence of 10 μM Y-27632 (Sigma), a well-characterized ROCK inhibitor, subcultured by trypsinization, and passaged 1:3 onto 100 mm culture dishes. The medium was changed every 72 hours. Rat chondrosarcoma (RCS) cells [13] and Rat2 cells were cultured at 37°C in DMEM supplemented with 10% FBS. The NP and AF cell morphology in monolayer cultures were observed microscopically. All experiments in this study were carried out with the recommendation in the Guide for the Care and Use of Laboratory Animals of the National Institute of Health, USA. The procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of Rush University Medical Center (Chicago).
Western blotting
After washing the cells twice with phosphate buffered saline (PBS), the cell lysates were prepared with RIPA buffer, (10 mM Tri-HCl, pH 7.4, 0.01% sodium dodecyl sulfate (SDS), and 0.1% Nonidet P-40 with protease inhibitors). The proteins in the lysates were separated by SDS-10% polyacrylamide gel electrophoresis and were transferred to a nitrocellulose membrane. The membranes were treated with the appropriate primary antibody and then with the appropriate secondary antibody labeled with horseradish peroxidase. The primary antibodies used were rabbit anti-SOX9 antibody (Millipore, Billerica, MA), rabbit anti-collagen I antibody (Abcam, Cambridge, MA), rabbit anti-β-actin antibody (Abcam), and mouse anti-β-catenin antibody (BD transduction). The horseradish peroxidase was detected using the ECL detection kit (Thermo Fisher Scientific, Rockford, IL).
Measurement of mRNA expression
To measure the mRNA expression, total RNA was extracted from primary NP and AF cells or NP and AF cells lines using RNeasy Plus Mini Kit (QIAGEN) according to the manufacturer's protocol. cDNA was prepared from the mRNA using iScript cDNA synthesis kit (Bio-rad). Quantitative polymerase chain reaction (qPCR) was performed using primers specific for each RNA, SYBR Master Mix, and a BIO-rad CFX96 system. The difference in Ct values (delta Ct) between the Ct value of each sample and that of GAPDH was calculated. The delta Ct value of each gene was then compared to that of each sample.
Gene expression analysis
Gene expression was analyzed by RT-PCR with specific genes that are known to play roles in proliferation and metabolism of NP and AF cells. A comparison of the mRNA expression of the specific genes for early and late passage cells was performed. Specific PCR primers used in this study are listed in Table 1.
Table 1.
Primer Sequences for PCR
| Primers | Forward (5’->3’) | Reverse (5’->3’) |
|---|---|---|
| Aggrecan | AGGATGGCTTCCACCAGTGC | TGCGTAAAAGACCTCACCCTCC |
| Col2a1 | CCTGGACCCCGTGGCAGAGA | CAGCCATCTGGGCTGCAAAG |
| Col1a1 | ATGTTCAGCTTTGTGGAC | GGATGCCATCTTGTCCAG |
| GAPDH | CCACAGTCCATGCCATCAC | TCCACCACCCTGTTGCTGTA |
| Sox9 | TAAATTCCCAGTGTGCATCC | GCACCAGGGTCCAGTCATA |
| Vimentin | CAGACAGGATGTTGACAATGC | GCTCCTGGATCTCTTCATCG |
| Laminin a5 | AGCTTTGTGATGGACAGTGG | CTTCTAGCCGCAGTGTGTTC |
| BSP1 | CGTCCAAGGAGACGCCCG | TGCTCGGAGCTGGCGACG |
| Mmp13 | GCAGCTCCAAAGGCTACAA | CATCATCTGGGAGCATGAAA |
| Col9a1 | CCTGCCAATTCTGTTTCTCA | AGGTCAAATCCTGGCAAGTC |
| β-catenin | GACAGAGTTGCTCCACTCCA | TGGCTTGTCCTCAGACATTC |
| rTERT | GTTGTCATCGAGCAGAGCAT | TTGCACATAGAACCTGCCAT |
| Col10a1 | GGGTAAAGAGATTTCAGTAAGAGGA | GTCCAGGACTTCCATAGCCT |
| TGF-β | CTTTGTACAACAGCACCCGC | TAGATTGCGTTGTTGCGGTC |
| IGF-1 | ATGAGCGCACCTCCAATAAAGA | ACGAACTGAAGAGCGTCCAC |
RESULTS
Morphology of Y-27632-immortalized NP and AF cells
NP and AF cells were isolated from the lumbar discs of 2-month-old adult rats. The cells were grown in the presence of 10 μM Y-27632 from day 3 onward. These cells continued to proliferate with more than 56 passages. In early passages, the NP and AF cells were actively dividing. The NP cells were isolated from gelatinous tissues and were cultured in monolayer with relatively small square-polygonal shape. The AF cell morphology under microscopy showed a uniformed fibroblast-like cell appearance (Fig. 1A, NP: P6 and AF: P3). After several passages, their characteristics were comparable with the early passage cell morphology (Fig. 1A, NP: P29 and AF: P33).
Figure 1. Representative phase-contrast images of Y-27632-immortalized NP and AF cells and telomerase expression increases over time.
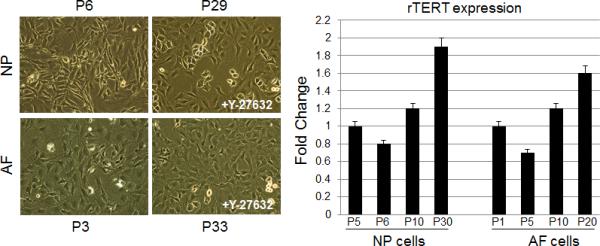
(A) Cells were expanded in monolayer culture and imaged at early passage (P2 of NP and AF) and late passage (P29 of NP and P33 of AF), respectively. (B) TERT mRNA levels from NP and AF cells, cultured in the presence of 10μM Y-27632 at the passage indicated, were quantitated by real-time RT-PCR. Each bar represents the mean of duplicated samples with standard deviation.
rTERT expression in Y27632-immortalized NP and AF cells
Telomerase reverse transcriptase (TERT) is the active subunit of telomerase, which maintains the telomere caps throughout multiple cell divisions of development [14]. Quantitative RT-PCR analysis showed the level of rTERT mRNA increased with the passaging of NP and AF cells in the presence of Y-27632 (Fig. 1B).
Cell proliferation
To determine changes in cell proliferation in Y-27632 immortalized NP and AF cells, we examined the expression of transforming growth factor-β (TGF-β) and insulin-like growth factor 1 (IGF-1) between early passage and late passage cells in NP and AF cells. We found that NP and AF cells continued to proliferate even at passage 60 in the presence of Y-27632. There was no significant increase in proliferation rate with Y-27632 treatment. TGF-β and IGF-1 have been shown to stimulate chondrocyte and endotheliocyte proliferation and matrix synthesis in vitro [15]. We found that the levels of TGF-β in NP and AF cells between early passage and late passage were not significantly changed, while the level of IGF-1 was significantly increased in the late passage of NP cells (Fig. 2A). Even though the rate of proliferation was not significantly increased in Y-27632 immortalized NP and AF cells, the growth rate of NP cells was faster than that of AF cells.
Figure 2. TGF-β and IGF-1 mRNA expression and specific marker gene expression profile in immortalized NP and AF cells.
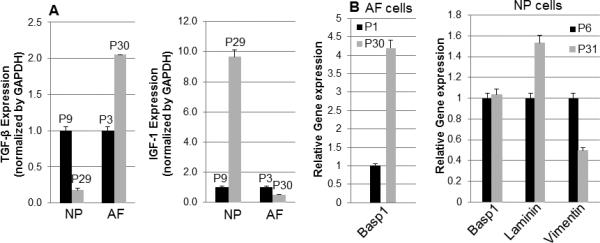
(A) Analyses of gene expression levels for TGF-β and IGF-1 were performed by quantitative RT-PCR. TGF-β and IGF-1 mRNA levels were compared between early passage and late passage cells with NP and AF cells, respectively. B. Basp1 (AF and NP) and Laminin (NP) mRNA level were examined for NP and AF marker analysis, but Vimentin mRNA level was detected for only NP marker analysis. The passage number of immortalized cells was indicated in graph. All experiments were normalized to GAPDH and statistical significance was assessed by Student t-test (p<0.05).
Specific marker gene expression of NP and AF cells after immortalization
To determine the maintenance of specific gene expression between early and late passages of NP and AF cells in the presence of Y-27632, gene expression of these cells was analyzed using RT-PCR. NP cells express phenotypic markers, including specific cytokeratins, vimentin, transcription factor (Brachyury T), cell surface marker (CD24), neuronal related protein (Basp) 1 and laminin [16-18]. NP and AF cells demonstrated no significant increase in Basp1 expression between cells at early and late passages. Of these markers, vimentin expression decreased at P31 in NP cells, but mRNA levels of these genes were not significantly different. In addition, laminin expression was not significantly changed in NP cells (Fig. 2B). These findings suggest that the ROCK inhibitor-induced cell immortalization could maintain their marker gene expression.
Extracellular matrix gene expression in NP and AF cells after immortalization
The healthy intervertebral disc contains hydrated and proteoglycan-rich NP tissue surrounded by fibrocartilagenous AF. The NP is an aggrecan-rich gel-like tissue [19] and the fibrocartilagenous outer AF tissue is composed of tightly packed collagen I fibrils and the inner AF tissue cells contains collagen type II, proteoglycans, and aggrecan [20-22]. NP cells are characterized by high expression for aggrecan and proteoglycan. To compare cells between early and late passages of NP and AF cells, we examined expression of collagen type II and aggrecan mRNA levels and found their expression was not significantly changed (Fig. 3A). In addition, we also examined expression of type I collagen and transcription factor Sox9, which is known to regulate collagen type II expression. As shown in Fig. 3A, Col1a1 and Sox9 mRNA expression was not significantly changed between early and late passages of NP cells. However, Col1a1 expression was enhanced (2-fold increase) in late passage of AF cells. mRNA levels of Col2a1, Sox9, and aggrecan were not changed in AF cells (Fig. 3B). Next we examined protein expression of Sox9, β-catenin, collagen type I and collagen type II by Western blotting. No differences were observed between early and late passages of NP or AF cells, confirming that late passage cells retain their phenotype (Fig. 3C). Interestingly, we observed difference of β-catenin localization in NP and AF cells. As shown in Fig. 4, β-catenin was expressed in the nucleus in NP cells but was expressed in the cytoplasma in AF cells.
Figure 3. Extracellular matrix gene expression profile.
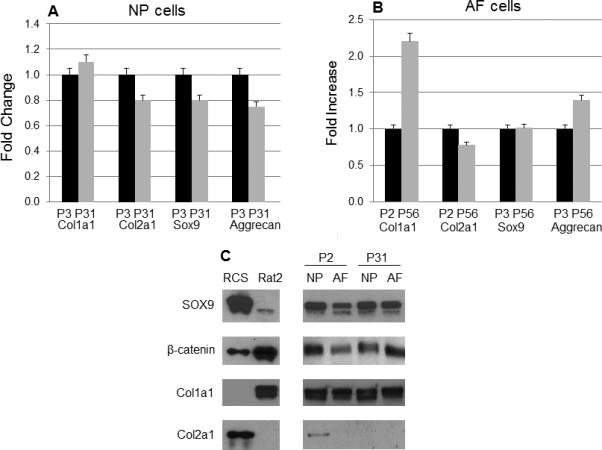
The majority of the genes expressed similar levels in immortalized NP and AF cells (A and B), but Col1a1 mRNA level was 2-fold increased in immortalized AF cells (B). (C) The protein levels of Sox9, β-catenin, Col1a1 and Col2a1 were analyzed on cell lysates from P2 or P31 of NP and AF cells by Western blotting. Equal sample loading was also confirmed by Ponceau S staining of the western blot membrane.
Data for a typical experiment are presented and experiments were performed more than three times from different passages with similar results.
Figure 4. Differential expression patterns of β-catenin protein in NP and AF cells.
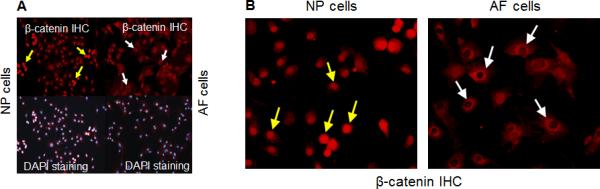
(A) β-catenin expession in NP cells was marked with yellow arrows and was shown as constitutively active form in nucleus but β-catenin expression was marked with white arrows in cytosolic parts in AF cells. Nuclei were stained with DAPI (blue). (B) Higher magnification IHC pictures show β-catenin protein expression in NP and AF cells.
Responses of NP and AF cells to cytokines after immortalization
To examine the responses of immortalized cells to cytokines, NP and AF cells were treated with 10 ng/ml of IL-1β for 24 hours. In NP cells, IL-1β strongly induced Mmp13 mRNA expression but did not induce β-catenin and Col10a1 mRNA expression. In AF cells, IL-1β did not increase β-catenin, Col10a1 but slightly increased Mmp13 mRNA expression (Fig. 5A). In contrast, Col1a1, Sox9 and aggrecan mRNA expression was decreased by the treatment of IL-1β in both NP and AF cells (Fig. 5B), suggesting that IL-1β regulated catabolic and anabolic genes in immortalized cells. Next, we examined whether TGF-β or BMP-2 regulates extracellular matrix production in immortalized NP and AF cells by treating cells with 10 ng/ml of TGF-β or BMP-2 for 24 hours. Interestingly, TGF-β increased Sox9 levels in both NP and AF cells with stronger effect in NP cells but decreased Mmp13 levels in AF cells (Fig. 5C). The increases in Co6l2a1 and aggrecan expression by BMP-2 were observed in NP but not AF cells (Fig. 5D).
Figure 5. The effect of cytokines on immortalized NP and AF cells.
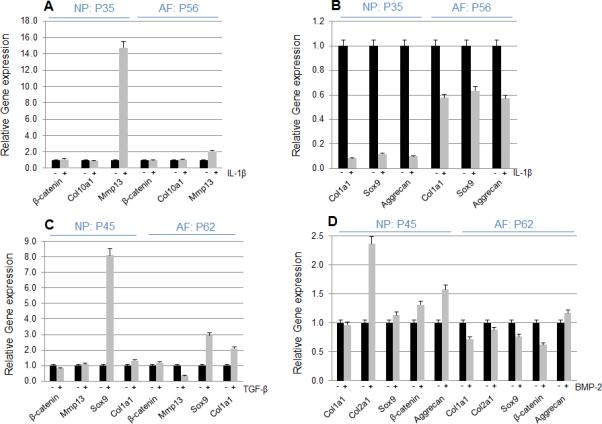
The NP and AF cells on late passage were cultured in the absence or presence of 10 ng/ml IL-1β or 10 ng/ml TGF-β or 100 ng/ml BMP-2 in normal medium for 24 hours. Relative gene expression analysis of β-catenin, Col10a1 and MMP13 was performed by quantitative RT-PCR (A). Col1a1, Sox9 and Aggrecan mRNA levels were decreased by the treatment with IL-1β for 24 hours in immortalized NP and AF cells (B). β-catenin, MMP13, Sox9 and Col1a1 mRNA levels were detected after treatment with TGF-β and Sox9 mRNA level was highly increased by TGF-β (C). BMP-2 increased Col2a1 mRNA level in NP cells and BMP-2 did not change the majority of genes in both NP and AF cells (D). Experiments were performed more than three times from different passages with similar results.
DISCUSSION
Disc degeneration is an important factor contributing to lower back pain in humans, but molecular mechanism remains unknown. One potential limitation for disc degeneration research is lacking proper cell lines behaving similar to primary disc cells. In the present studies we have established NP and AF cell lines from cells isolated from lumbar discs of rats using ROCK inhibitor, Y-27632. Rho kinase inhibition has been reported to affect kerainocyte proliferation and differentiation [23]. First, we have examined morphological characteristics. Primary disc cells can be split 10 to 15 passages with certain degree morphological changes. In contrast, immortalized NP and AF cells could be passed over 50 passages without losing their proliferation and phenotypic properties. The morphology of NP cells is more like chondrocytes as compared to AF cells and we have not detected the significant phenotypic differences when comparing early passage NP cells with late passage cells. We determined changes in TERT in immortalized NP and AF cells and found that TERT mRNA expression was upregulated in NP and AF cells to maintain the telomere caps throughout the multiple cell divisions. It is possible that immortalized cells may change their property and function comparing to the primary cells. We therefore examined the cell proliferation. The proliferation rate of immortalized NP and AF cells was not decreased after serial passages. TGF-β and IGF-1 are known to stimulate NP cell proliferation [24]. In the present studies, TGF-β was upregulated in late passage of AF cells but IGF-1 was upregulated in late passage of NP cells. Specific NP and AF cell markers have been reported in human NP and AF cells. Basp1 and laminin have been suggested as NP and AF cell markers but vimentin was suggested as a specific NP cell marker. Our immortalized cell line showed the consistency in terms of marker gene expression. BSP1 expression was increased in late passage of AF cells. Further investigation is required to determine the role of this gene in AF cells. Although some gene expressions are upregulated in immortalized cells, extracellular matrix gene expression was not significantly changed between early and late passage of cells. Previous reports showed that disc cells produced large amounts of proteoglycan and collagen type II. However, our immortalized cell lines expressed mRNA of collagen type I and type II, but the protein of collagen type II was only weakly expressed in early passage of NP cells but not expressed in late passage of NP cells and in early and late passages of AF cells. These were demonstrated by Western blotting (Fig. 4C) and IHC (data not shown). In this study, we used monolayer cell culture approach which may affect collagen expression. In future studies, we will apply 3D cell culture method for our immortalized NP and AF cells.
In addition, we detected transcription factors Sox9 and β-catenin expression in NP and AF cells and we did not find significant differences in Sox9 and β-catenin expression in early and late passages of NP and AF cells. Using IHC assay, we found that β-catenin protein was mainly localized in the nucleus of NP cells; in contrast, β-catenin protein was mostly localized in cytosolic portion of AF cells. These results suggest that functions of NP and AF cells are different. NP cells have constitutively active β-catenin signaling, but AF cells may be more responsive to growth factor signaling and stimulation. β-catenin was reported to play an important role in cell proliferation, differentiation and apoptosis in chondrocytes [25, 26]. Further studies are needed to determine differential roles of β-catenin in NP and AF cells. Next, we examined the effect of anabolic and catabolic cytokines in immortalized NP and AF cells. Previous reports demonstrated that human NP and AF cells constitutively express NGF mRNA and protein and proinflammatory cytokines IL-1β stimulated NGF production [27]. Wang et al. [28] reports that TGFβ-1 and IL-1β are involved in the synthesis and degradation for the extracellular matrix and may act as potential therapeutic targets for the prevention or treatment of disc degeneration. Our results showed IL-1β increased Mmp13 in NP cells but decreased matrix genes and transcription factor Sox9 in both of NP and AF cells. In addition, NP cells seem more sensitive to the treatment with IL-1β. TGF-β increased Sox9 expression in NP and AF cells but BMP-2 only increased collagen type II in NP cells. These results suggest that NP and AF cells have distinct properties in responses to different cytokines. More detail investigations are still required to further characterize these immortalized NP and AF cells.
Acknowledgments
The manuscript submitted does not contain information about medical device(s)/drug(s). NIH grants AR055915 and AR054465 and North American Spine Society (NASS) grant funds were received in support of this work. Relevant financial activities outside the submitted work: board membership, consultancy, grants, royalties, stocks.
References
- 1.Bogduk N. The lumbar disc and low back pain. Neurosurg Clin N Am. 1991;2:791–806. [PubMed] [Google Scholar]
- 2.Luoma K, Riihimaki H, Luukkonen R, et al. Low back pain in relation to lumbar disc degeneration. Spine. 2000;25:487–92. doi: 10.1097/00007632-200002150-00016. [DOI] [PubMed] [Google Scholar]
- 3.Urban JP, Roberts S. Degeneration of the intervertebral disc. Arthritis Res Ther. 2003;5:120–30. doi: 10.1186/ar629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feng H, Danfelter M, Stromqvist B, et al. Extracellular matrix in disc degeneration. J Bone Joint Surg Am. 2006;88:25–9. doi: 10.2106/JBJS.E.01341. [DOI] [PubMed] [Google Scholar]
- 5.Pattappa G, Li Z, Peroglio M, et al. Diversity of intervertebral disc cells: phenotype and function. J Anat. 2012;221:480–96. doi: 10.1111/j.1469-7580.2012.01521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao CQ, Wang LM, Jiang LS, et al. The cell biology of intervertebral disc aging and degeneration. Ageing Res Rev. 2007;6:247–61. doi: 10.1016/j.arr.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 7.Liu MC, Chen WH, Wu LC, et al. Establishment of a promising human nucleus pulposus cell line for intervertebral disc tissue engineering. Tissue Eng Part C Methods. 2014;20:1–10. doi: 10.1089/ten.TEC.2013.0048. [DOI] [PubMed] [Google Scholar]
- 8.Liang W, Ye D, Dai L, et al. Overexpression of hTERT extends replicative capacity of human nucleus pulposus cells, and protects against serum starvation-induced apoptosis and cell cycle arrest. J Cell Biochem. 2012;113:2112–21. doi: 10.1002/jcb.24082. [DOI] [PubMed] [Google Scholar]
- 9.Sakai D, Mochida J, Yamamoto Y, et al. Immortalization of human nucleus pulposus cells by a recombinant SV40 adenovirus vector: establishment of a novel cell line for the study of human nucleus pulposus cells. Spine. 2004;29:1515–23. doi: 10.1097/01.brs.0000131419.25265.23. [DOI] [PubMed] [Google Scholar]
- 10.Chapman S, Liu X, Meyers C, et al. Human keratinocytes are efficiently immortalized by a Rho kinase inhibitor. J Clin Invest. 2010;120:2619–26. doi: 10.1172/JCI42297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu X, Ory V, Chapman S, et al. ROCK inhibitor and feeder cells induce the conditional reprogramming of epithelial cells. Am J Pathol. 2012;180:599–607. doi: 10.1016/j.ajpath.2011.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ellman MB, Kim JS, An HS, et al. The pathophysiologic role of the protein kinase Cdelta pathway in the intervertebral discs of rabbits and mice: in vitro, ex vivo, and in vivo studies. Arthritis Rheum. 2012;64:1950–9. doi: 10.1002/art.34337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mukhopadhyay K, Lefebvre V, Zhou G, et al. Use of a new rat chondrosarcoma cell line to delineate a 119-base pair chondrocyte-specific enhancer element and to define active promoter segments in the mouse pro-alpha 1(II) collagen gene. J Biol Chem. 1995;270:27711–9. doi: 10.1074/jbc.270.46.27711. [DOI] [PubMed] [Google Scholar]
- 14.Harley CB, Futcher AB, Greider CW. Telomeres shorten during ageing of human fibroblasts. Nature. 1990;345:458–60. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- 15.Delatte ML, Von den Hoff JW, Nottet SJ, et al. Growth regulation of the rat mandibular condyle and femoral head by transforming growth factor-{beta}1, fibroblast growth factor-2 and insulin-like growth factor-I. Eur J Orthod. 2005;27:17–26. doi: 10.1093/ejo/cjh068. [DOI] [PubMed] [Google Scholar]
- 16.Chen J, Lee EJ, Jing L, et al. Differentiation of mouse induced pluripotent stem cells (iPSCs) into nucleus pulposus-like cells in vitro. PLoS One. 2013;8:e75548. doi: 10.1371/journal.pone.0075548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tang X, Jing L, Chen J. Changes in the molecular phenotype of nucleus pulposus cells with intervertebral disc aging. PLoS One. 2012;7:e52020. doi: 10.1371/journal.pone.0052020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sakai D, Nakamura Y, Nakai T, et al. Exhaustion of nucleus pulposus progenitor cells with ageing and degeneration of the intervertebral disc. Nat Commun. 2012;3:1264. doi: 10.1038/ncomms2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sive JI, Baird P, Jeziorsk M, et al. Expression of chondrocyte markers by cells of normal and degenerate intervertebral discs. Mol Pathol. 2002;55:91–7. doi: 10.1136/mp.55.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gruber HE, Leslie K, Ingram J, et al. Cell-based tissue engineering for the intervertebral disc: in vitro studies of human disc cell gene expression and matrix production within selected cell carriers. Spine J. 2004;4:44–55. doi: 10.1016/s1529-9430(03)00425-x. [DOI] [PubMed] [Google Scholar]
- 21.Bron JL, Helder MN, Meisel HJ, et al. Repair, regenerative and supportive therapies of the annulus fibrosus: achievements and challenges. Eur Spine J. 2009;18:301–13. doi: 10.1007/s00586-008-0856-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singh K, Masuda K, Thonar EJ, et al. Age-related changes in the extracellular matrix of nucleus pulposus and anulus fibrosus of human intervertebral disc. Spine. 2009;34:10–6. doi: 10.1097/BRS.0b013e31818e5ddd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Terunuma A, Limgala RP, Park CJ, et al. Efficient procurement of epithelial stem cells from human tissue specimens using a Rho-associated protein kinase inhibitor Y-27632. Tissue Eng Part A. 2010;16:1363–8. doi: 10.1089/ten.tea.2009.0339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gruber HE, Fisher EC, Jr., Desai B, et al. Human intervertebral disc cells from the annulus: three-dimensional culture in agarose or alginate and responsiveness to TGF-beta1. Exp Cell Res. 1997;235:13–21. doi: 10.1006/excr.1997.3647. [DOI] [PubMed] [Google Scholar]
- 25.Day TF, Guo X, Garrett-Beal L, et al. Wnt/beta-catenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte differentiation during vertebrate skeletogenesis. Dev Cell. 2005;8:739–50. doi: 10.1016/j.devcel.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 26.Akiyama H, Lyons JP, Mori-Akiyama Y, et al. Interactions between Sox9 and beta-catenin control chondrocyte differentiation. Genes Dev. 2004;18:1072–87. doi: 10.1101/gad.1171104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abe Y, Akeda K, An HS, et al. Proinflammatory cytokines stimulate the expression of nerve growth factor by human intervertebral disc cells. Spine. 2007;32:635–42. doi: 10.1097/01.brs.0000257556.90850.53. [DOI] [PubMed] [Google Scholar]
- 28.Wang SL, Yu YL, Tang CL, et al. Effects of TGF-beta1 and IL-1beta on expression of ADAMTS enzymes and TIMP-3 in human intervertebral disc degeneration. Exp Ther Med. 2013;6:1522–6. doi: 10.3892/etm.2013.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]


