Abstract
Background
Few studies have detailed the prenatal pesticide exposure levels of women employed in or residing near large-scale agricultural industries. This study reports pesticide metabolite levels during and shortly after pregnancy in a pilot study of workers in Ecuador.
Methods
Urine samples were collected for 16 rose workers and 10 non-agricultural workers enrolled into the study in early pregnancy. We measured six nonspecific organophosphate dialkylphosphate (DAP) pesticide metabolites, two alkylenebis-dithiocarbamate pesticide metabolites (ethylene thiourea [ETU] and propylene thiourea [PTU]), 3,5,6-trichloro-2-pyridinol [TCPy], malathion dicarboxylic acid, and two pyrethroid metabolites (2,2-dimethylcyclopropanecarboxylic acid and 3-phenooxybenzoic acid).
Results
We collected 141 urine samples (mean: 5.4 per woman). We observed high detection frequencies for five DAP metabolites and ETU, PTU, and TCPy. We report elevated levels of ETU in the entire sample (median 4.24 ng/mL, IQR 2.23, 7.18), suggesting other possible non-occupational pathways of exposure. We found no statistical differences in pesticide levels by current employment status, though the highest pesticide levels were among rose workers. We observed within-woman correlation in TCPy and PTU levels, but not in ETU or DAP levels.
Conclusions
The present study is the first to characterize prenatal pesticide exposure levels among working women in Ecuador. Limitations include a small sample size and use of a convenience sample. Strengths include a longitudinal design and multiple urine samples per woman. Results provide an initial characterization of prenatal pesticide exposure levels and how these levels vary over pregnancy in a community impacted by agricultural industry and will inform further studies in the region.
1. Introduction
Industrial agriculture is an increasingly important component of the economies of developing countries, accounting for a large percentage of the production of goods for use in the United States and other industrialized countries. These industries also account for a large percentage of global pesticide use (Alavanja 2009; Ecobichon 2001; World Health Organization 1990). Non-persistent pesticides, which include the organophosphate (OP), carbamate, and pyrethroid classes of pesticides, have become a widely used alternative to persistent pesticides such organochlorines. This is especially the case in large-scale export-led agricultural production. Research indicates that prenatal exposure to non-persistent pesticides are associated with central nervous system teratogenicity and can cause deficits in neurobehavioral development (Eskenazi et al. 2008; Grandjean and Landrigan 2014; Jurewicz and Hanke 2008; Rice and Barone 2000; Rosas and Eskenazi 2008; Scheuplein et al. 2002).
Worldwide, young women of reproductive age have become an integral part of the labor force of corporate agricultural industry (World Bank 2005; Hallam et al. 2004). Few studies have detailed the prenatal pesticide exposure levels of women employed in these industries (London et al. 2002). The majority of studies that assess urinary pesticide metabolite levels in pregnancy have been conducted in industrialized countries (Berman et al. 2011; Engel et al. 2007; Eskenazi et al. 2007; Huen et al. 2012; Llop et al. 2013; Rauh et al. 2006; Whyatt et al. 2002; Ye et al. 2009; Zhang et al. 2014) Very few biomonitoring studies documenting prenatal non-persistent pesticide exposure levels in working female populations have been conducted in the developing world. Even fewer have focused on exposures to the pregnant laborer residing in resource poor countries.
The Ecuadorian cut-flower industry is a key example of a growing agricultural export industry. This industry has become the country’s third most important export, with an annual increase 11% from 2001–2011. In 2010, approximately $610 million of cut-flowers were exported, with roses comprising $440 million of that total (Direccion de Inteligencia Comercial e Inversiones 2011). The geographic location of Ecuador provides optimal conditions for year-long cut-flower production. While the international demand for cut-flowers, particularly roses, has some seasonality (i.e., peak production periods occur several months before St. Valentine’s Day and Mother’s Day), the flower industry in Ecuador is unique in that production occurs all year long. Approximately 60%-70% of the work force in this industry is female. Female flower laborers mainly work in cultivation or post-harvest, typically working 6 days/week, with longer schedules during peak seasons (Sawers 2005). If they become pregnant, many continue working during their entire pregnancy. Pesticide use in this industry is common and widespread, with the most commonly used classes of pesticides being the organophosphates, dithiocarbamates, and pyrethroids.
In 2003, we conducted a study of the relationship between maternal employment in the flower industry and the neurodevelopment of young children living in a major flower-growing region (Handal et al. 2008; Handal et al. 2007a; Handal et al. 2007b; Handal et al. 2007c). This study was limited by its cross-sectional design and the use of questionnaire exposure measures that had not been validated with any type of biomarker exposure measures during pregnancy. Building on this research, we conducted a study to assess the feasibility and acceptability of urine collection methods; and conduct a preliminary characterization of pesticide exposure levels during pregnancy in a population of women working in a flower growing region of Ecuador– something that, to date, had never been done.
The present paper characterizes and quantifies pesticide exposure levels of pregnant women participating in the pilot birth cohort study. The objectives of this analysis were to report overall pesticide and metabolite levels during and shortly after pregnancy, to assess differences between rose farm workers and women working in non-agricultural occupations who reside in the same region, and to assess intra-woman variability across pregnancy for this cohort of pregnant Ecuadorian women.
2. Materials and Methods
2.1 Study population and enrollment
Two specific regions in the Pichincha Province of Ecuador, Cayambe and Pedro Moncayo, have seen a dramatic increase in greenhouse cut-flower production, with the majority dedicated to long-stem rose production. Our pilot study focused on a population of working pregnant women living in this region. Specific study population details have been published elsewhere (Handal et al. 2014). Briefly, eligible women were in their first or second trimester of pregnancy. Because this was the first study conducted in the region quantifying prenatal pesticide exposure levels and we did not have a good sense of the background exposures, we recruited two groups for the pilot study: 1) women who were currently employed in the rose industry during their pregnancy and 2) women who were currently working full-time outside of the home for pay, but who had not worked in any agricultural industry in the past five years. All women had to have resided in the region for at least one year prior to enrollment. The target enrollment number was 30 women: 20 pregnant rose workers and 10 pregnant non-agricultural workers.
Recruitment and enrollment occurred over a period of 10 months in 2011. The study team initially identified potential participants during prenatal visits at local clinics and then used snowball sampling techniques for identifying more eligible women. The study team worked closely with a Community Advisory Board in all phases of the study, which aided in the enrollment and follow-up of the study participants. Approval for this project was obtained from the Institutional Review Boards at the University of New Mexico Health Sciences Center and the University San Francisco de Quito in Cumbaya, Ecuador. Informed consent was obtained.
2.2 Exposure data collection
The study team collected biospecimens and conducted interviews at enrollment and at pregnancy follow-ups every 4–6 weeks, and a follow-up approximately 7–9 days after delivery. During the interviews, participants were administered a questionnaire assessing occupational and home sources of pesticide exposure, work characteristics, maternal health characteristics, and other lifestyle and socio-demographic factors.
At each visit including enrollment, urine samples were collected at the participant’s home to ensure privacy and increase the likelihood of participation. After initial training with the study team, participants were asked to obtain the first void of the morning using a sterile container provided by the study team the morning of the scheduled follow-up interview. The sample was collected in a 150 mL sterile flask and refrigerated until after the interview was completed, at which time the study nurse immediately transferred the sample to the Raúl Maldonado Mejia Hospital in Cayambe in a cooler filled with cold gels. Study staff homogenized the sample and with aid of a glass Pasteur pipette, filled three red top vacutainer tubes with approximately 7 mL of the sample. The three tubes were labeled and immediately stored at −20°C until shipped on dry ice to Emory University. The number of urine samples for each woman in the cohort depended on the time of enrollment. Between 3 and 7 longitudinal samples were collected for each woman, with an average of 5.4 samples per woman.
2.3 Sample analysis
Floriculture depends highly on the use of a variety of pesticides particularly insecticides and fungicides. High quantities and a constant variety of chemicals must be used to ensure that the final product be blemish-free and be able to reach the market in peak condition. All of these factors along with the lack of organized unions in the Ecuadorian flower industry and the lack of publicly available pesticide use information make characterization of pesticides a challenge. Based on previous work done in the region (Breilh 2007; Breilh et al. 2005; Colosio et al. 2003; Harari et al. 2004), we analyzed metabolite levels for a range of the most commonly used classes of pesticides in the industry: organophosphates, bis-dithiocarbamates, and pyrethroids. Urine samples were analyzed for six non-specific OP dialkyl phosphate (DAP) pesticide metabolites including three dimethyl phosphates – dimethylphosphate (DMP), dimethyldithiophosphate (DMDTP), and dimethylthiophosphate (DMTP); and three diethyl phosphates – diethylphosphate (DEP), diethyldithiophosphate (DEDTP), and diethylthiophosphate (DETP). Samples were also analyzed for two alkylenebis-dithiocarbamate pesticide metabolites (ethylenethiourea [ETU] and proplylenethiourea [PTU]), and two other OP pesticide metabolites (3,5,6-trichloro-2-pyridinol [TCPy], malathion dicarboxylic acid [MDA]), and pyrethroid pesticide metabolites (2,2-dimethylcyclopropanecarboxylic acid [DCCA] and 3-phenooxybenzoic acid [3-PBA]) using established protocols (Montesano et al. 2007; Olsson et al. 2004; Prapamontol et al. 2014). Briefly, aliquots of urine were spiked with isotopically labeled internal standards. Samples were extracted and concentrated. Concentrated samples were analyzed using high performance liquid chromatography-tandem mass spectrometry or gas chromatography-tandem mass spectrometry. The limits of detection (LOD) ranged from 0.1 to 0.5 ug/L, with relative standard deviations less than 12%. The individual pesticides are left-censored data, where censoring occurs at the limit of defection.
Urine data were not creatinine adjusted because of the physiologic changes in pregnancy that increase the intra-person variation of creatinine excretion thus rendering it unsuitable for normalizing urine dilution (Bradman et al. 2005). Because the DAP metabolites devolve from diethyl- and dimethyl-substituted OP pesticides, we converted each metabolite into their molar concentrations and summed to create three summary measures: Total DE representing the sum of the three metabolites derived from diethyl-substituted OPs; Total DM representing the sum of the three metabolites derived from dimethyl-substituted OPs; and Total DAP representing the sum of all six metabolites. When DAPS were below the limit of detection (LOD), we imputed LOD/sqrt(2) for the purpose of constructing these three summary DAP measures (Barr et al. 1999; Barr et al. 2004; CDC 2003).
2.4 Statistical analysis
To describe the study cohort, we present demographic and maternal characteristic summary measures at enrollment for maternal age, weeks pregnant at the enrollment survey, self-reported health, marital status, maternal ethnicity, maternal education level, monthly household income, and home ownership, stratified by type of work. Pesticide measures were available for all 26 women at enrollment; we collected 90 samples during pregnancy following enrollment, for a total of 116 prenatal samples among the 26 women. Pesticide levels were measured for 25 women at a time point shortly after delivery (one woman dropped out of the study after delivery).
First, we summarized pesticide levels using basic summary statistics. To calculate these statistics, we imputed LOD/sqrt(2) for measures below the limit of detection. We then summarized pesticide levels using geometric means, medians, 90th percentiles, maxima, and the percent above the LOD. We descriptively summarized pesticide levels for enrollment versus delivery samples for all women in the study; we also descriptively summarized pesticide levels by current employment status across all prenatal samples to crudely compare pesticide levels between those employed in the rose industry and other non-agricultural working women. We define the current employment indicator as having two categories: currently working in the rose-industry at the time of urine collection (referred to as “RW”) and working in other non-agricultural occupations or no longer working at the time of urine collection (referred to as “Others”).
We fit regression models to the data to assess the following questions of interest: (1) How do pesticide levels vary over time? Specifically, are there temporal trends in pesticide levels over the course of the pregnancy? And, are pesticide levels lower following delivery compared to enrollment?; (2) Is there a difference in pesticide levels between current rose-workers (RW) and those not currently working in the rose-industry (Others)?; and (3) Are pesticide levels correlated within-woman over the course of her pregnancy, after controlling for weeks of pregnancy and current employment in the rose industry?
To address these questions, we modeled the log-pesticide levels using mixed effects regression models with subject-level random effects, allowing for within-individual correlation in pesticide levels over time. The individual pesticide measures were left-censored, whereas the summary DAP measures were not (because we imputed for the individual censored pesticides to construct the summary measure). We used a linear mixed effects model for the summary DAP measures. Very few of the individual metabolites were below the LOD for the DMP and DAP measures; therefore, imputing LOD/sqrt(2) for the individual metabolites should not impact the results. However, censoring frequencies were high (>50%) for all of the individual DEP measures; we therefore did not model Total DEP.
Rather than imputing LOD/sqrt(2) for the individual measures in the regression model, which has known limitations (Lubin et al. 2004), we use regression methods appropriate for censored data. Specifically, to account for the left-censored and longitudinal nature of the individual pesticide data, we ran a Tobit regression model, which is a latent variable model that accommodates censored observations by modeling the latent unobserved outcome for the censored measurement as a normal random variable conditional on a set of covariates (Lubin et al. 2004; Tobin 1958). We ran a small simulation study to ensure that the Tobit regression model would produce unbiased inferences within the small sample size. The simulation results suggest that inferences for the fixed effects are unbiased even with the small sample size and up to 50% left censoring (standard errors were underestimated for higher levels of censoring). Subsequently, we did not model any pesticides with > 50% of the observations below the LOD; high levels of censoring suggest that these are not common exposure routes in the study population. The left-censored pesticides that we modeled in the analysis were: TCPy, ETU, PTU, and DMTP.
To address question 1): How do pesticide levels vary over time, and specifically, are there temporal trends in pesticide levels over the course of the pregnancy?,” we fit the models to only the prenatal samples (n=116), including weeks pregnant as the explanatory variable in the model. We then estimated the multiplicative change in geometric mean pesticide levels from 20 to 34 weeks, because these are the midpoints of the second and third trimesters and represent time points that fall within the range of our collected data. We tested for a temporal trend in pesticide levels over pregnancy using the regression coefficient for weeks pregnant. Additionally, to address the question “Are pesticide levels lower following delivery compared to enrollment?,” we modeled the pesticide levels at enrollment and delivery time points only and included time point in the model (n=50), excluding the one woman who dropped out at delivery from the modeling. We estimated the multiplicative change in the geometric mean pesticide levels from enrollment to delivery and tested for a change from enrollment to delivery using the regression coefficient for time point. Results from these regression models were similar after adjusting for current employment and are not shown.
To address question 2): “Is there a difference in pesticide levels between current rose-workers (RW) and those not currently working in the rose-industry (Others)?,” we fit the models to only the prenatal samples (n=116), including RW/Other status at the time of urine collection in the model. We estimated the multiplicative change in the geometric mean pesticide levels between rose workers and other workers and tested for a change between RW/Other using the regression coefficient for time point. To address question 3): Are pesticide levels correlated within each woman, after controlling for weeks of pregnancy and current employment in the rose industry?,” we fit regression models including weeks pregnant and current employment and estimated the intraclass correlation (ICC) from the estimated variance components from these models. The ICC reflects the magnitude of the estimated within-subject and between-subject variance across pregnancy using the intraclass correlation (ICC)
Lastly, to assess potential exposure to chlorpyrifos (commonly used for mosquito control in rose production), we calculated the Spearman correlation between TCPy and DETP, between TCPy and DEP, and between DEP and DETP. We performed this analysis on all samples (n=141); and restricting to women with > LOD levels of all three of these pesticide metabolites (n=39); and tested the null that these correlations were equal to 0.
We analyzed the data using a complete-case analysis for the pesticide metabolite concentration measurements (using all available measurements). There were no missing data for the demographics, weeks of pregnancy, or current employment status. All logarithms refer to the natural log. All hypothesis tests were two-sided and conducted at the 0.05 level of significance (unless otherwise stated) and confidence intervals were at the 95% level. We did not correct p-values for multiple comparisons. All analyses were conducted in Stata v13 (StataCorp LP, College Station, TX).
3. Results
3.1 Socio-demographic and maternal characteristics
Data were collected on 16 rose workers and 10 comparison workers, all of whom worked outside of the home for pay. Out of the 26 enrolled mothers, 25 completed the study while one was lost to follow-up prior to the post-delivery interview and subsequent four-month maternal-infant post-partum follow-up interview. A total of 141 urine samples were obtained: 116 were taken during pregnancy and 25 were taken at post-delivery. Of the 116 pregnancy urine samples, 4% were collected during the first trimester, 52% during the second trimester, and 44% during the third trimester. Eighty-one percent of women reported working after 30 weeks of pregnancy.
Table 1 shows the socio-demographic and maternal characteristics by exposure group. The mean age of the total population at enrollment was 27.1 years, and participants were, on average, in mid-second trimester of pregnancy at enrollment. Rose workers on average reported less education; 50% of rose-workers had at most a primary education, compared to 10% of the other workers. Observed reported monthly household incomes were slightly higher for rose workers. Additionally, 44% of rose-workers owned their home compared to 20% of the other workers, who also reported more economic difficulties in meeting household food needs.
Table 1.
Socio-demographic and maternal characteristics of the study population, by occupational group, at enrollment (N=26). Cayambe-Pedro Moncayo region of Ecuador. 2010–2012
| Characteristic | Rose workers (n = 16) |
Other workers (n = 10) |
P-value |
|---|---|---|---|
| Age (mean ± SD) | 28.4 ± 6.2 | 25.1 ± 5.1 | 0.15 |
| Weeks pregnant (mean ± SD) | 17 ± 4.1 | 19.4 ± 5.4 | 0.2 |
| n (%) | n (%) | ||
| Maternal Education | 0.02* | ||
| Primary (incomplete /complete) | 8 (50) | 1 (10) | |
| Secondary (incomplete/ complete) | 7 (44) | 4 (40) | |
| University (incomplete /complete) | 1 (6) | 5 (50) | |
| Marital Status | 0.64 | ||
| Married /living as married civil union | 13 (81) | 7 (70) | |
| Maternal Ethnicity | 0.42 | ||
| Indigenous | 6 (38) | 2 (20) | |
| Mestizo /White | 10 (62) | 8 (80) | |
| Self-reported health | |||
| Good or excellent | 5 (31) | 5 (50) | 0.42 |
| Monthly Household Income | |||
| Income > $400 | 10 (63) | 5 (50) | 0.69 |
| Economic difficulties | |||
| Cut back on children’s food in last week | 3 (19) | 3 (38)a | 0.36 |
| Cut back on adults’ food in last week | 4 (25) | 5 (50) | 0.23 |
| Home Ownership | |||
| Owns own home | 7 (44) | 2 (20) | 0.40 |
Notes: SD, standard deviation
P-value < 0.05
n=8
3.2 Dialkyl phosphate (DAP) metabolites
Table 2a presents the summary statistics for the six non-specific DAP metabolites, total DAP measures, by sampling time point (enrollment and delivery). Detection frequencies were the highest (>80%) for the DMTP metabolite, at both sampling time points. With the exception of the DMDTP metabolite, detection frequencies and geometric means for all dimethyl and diethyl metabolites were higher for the enrollment sample (collected at approximately 18 weeks gestation) compared to the delivery sample (collected at approximately 9 days after delivery). Overall, DM metabolite levels were, on average, higher than DE metabolites for the total study population, at both time points.
Table 2.
Summary of urinary metabolite concentrations at enrollment and delivery, Cayambe-Pedro Moncayo region of Ecuador. 2010–2012
| 2a. | |||||||
|---|---|---|---|---|---|---|---|
| DAP metabolites | Samplea | LOD (ug/L) |
DF | Geo. Mean | 50th | 90th | Max |
| Total DAP (nmol/L) | Enrollment | -- | -- | 83.64 | 83.99 | 215.29 | 756.18 |
| Delivery | -- | -- | 43.14 | 40.01 | 146.72 | 263.53 | |
| Total DM (nmol/L) | Enrollment | -- | -- | 51.56 | 51.61 | 179.11 | 577.04 |
| Delivery | -- | -- | 34.52 | 35.55 | 120.22 | 181.82 | |
| DMP (ng/mL) | Enrollment | 4.80 | 0.31 | 7.87 | 3.41 | 58.45 | 165.89 |
| Delivery | 4.80 | 0.03 | 6.06 | 3.41 | 28.95 | 62.90 | |
| DMTP (ng/mL) | Enrollment | 1.42 | 0.92 | 35.41 | 40.62 | 138.31 | 250.63 |
| Delivery | 1.42 | 0.84 | 19.76 | 24.46 | 75.42 | 118.28 | |
| DMDTP (ng/mL) | Enrollment | 0.64 | 0 04 | 0.56 | 0.45 | 0 45 | 160.52 |
| Delivery | 0.64 | 0 08 | 0.62 | 0.45 | 0.45 | 30.77 | |
| Total DE (nmol/L) | Enrollment | -- | -- | 8.26 | 1.71 | 171.96 | 710.51 |
| Delivery | -- | -- | 3.27 | 1.71 | 35.49 | 147.75 | |
| DEP (ng/mL) | Enrollment | 1.31 | 0.42 | 5.07 | 0.92 | 90.44 | 291.44 |
| Delivery | 1.31 | 0.20 | 1.82 | 0.92 | 27.68 | 78.18 | |
| DETP (ng/mL) | Enrollment | 0.59 | 0.31 | 1.41 | 0.41 | 44.95 | 214.29 |
| Delivery | 0.59 | 0.16 | 0.74 | 0.41 | 9.76 | 39.98 | |
| DEDTP (ng/mL) | Enrollment | 0.54 | 0.12 | 0.69 | 0.38 | 36.57 | 204.77 |
| Delivery | 0.54 | 0 04 | 0.45 | 0.38 | 0.38 | 29.58 | |
| 2b. | |||||||
|---|---|---|---|---|---|---|---|
| Other metabolites (ng/mL) |
|||||||
| TCPy | Enrollment | 0.1 | 1.00 | 1.25 | 1.06 | 4.70 | 8.52 |
| Delivery | 0.1 | 0.60 | 0.37 | 0.64 | 1 63 | 3.90 | |
| DCCA | Enrollment | 0.1 | 0 04 | 0.09 | 0.07 | 0.07 | 16.59 |
| Delivery | 0.1 | 0.00 | 0.07 | 0.07 | 0.07 | 0.07 | |
| 3PBA | Enrollment | 0.1 | 0.27 | 0.12 | 0.07 | 0.39 | 3.93 |
| Delivery | 0.1 | 0.52 | 0.16 | 0.14 | 0.51 | 3.56 | |
| PTU | Enrollment | 0.1 | 0.81 | 0.59 | 0.87 | 1.94 | 6.30 |
| Delivery | 0.1 | 0.88 | 0.78 | 0.85 | 3.02 | 4.44 | |
| ETU | Enrollment | 0.1 | 1.00 | 3.63 | 3.80 | 10.41 | 23.09 |
| Delivery | 0.1 | 0 96 | 3.73 | 4.56 | 12.29 | 74.23 | |
Abbreviations: OP, organophosphate; LOD, limit of detection; DF, detection frequency; Geo. Mean, Geometric Mean; Max, maximum.
Enrollment sample was collected at approximately 18 weeks gestation with n = 26 samples Delivery sample was collected at approximately 9 days post-partum with n = 25 samples.
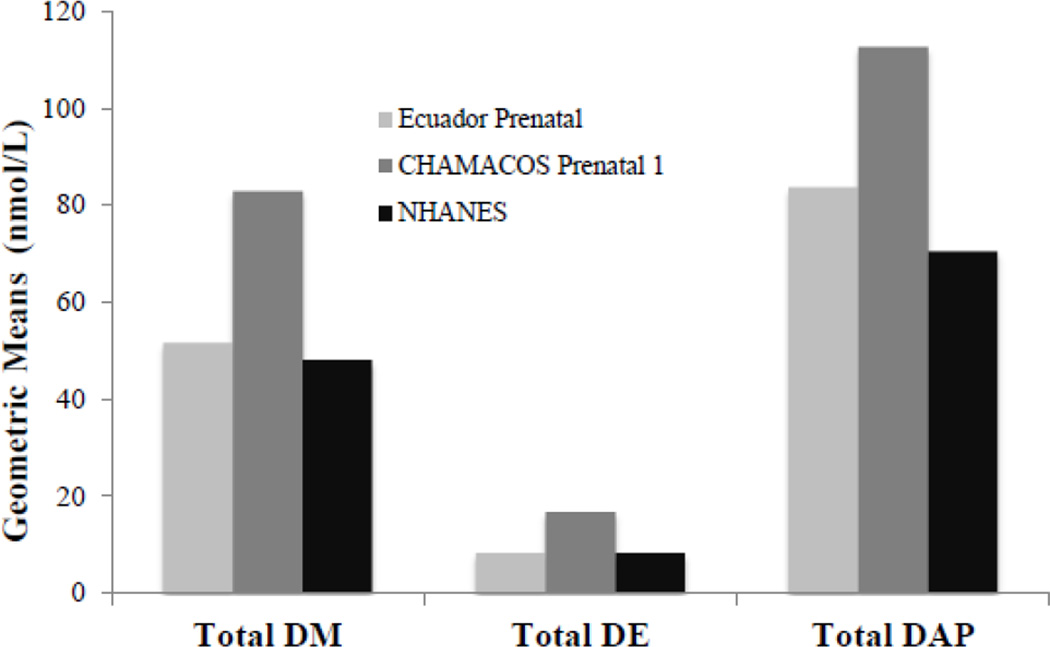
To graphically compare our study population to existing cohorts, we compared our cohort’s geometric mean summary DAP levels at enrollment to those reported from other cohorts. Figure 1 presents a comparison of our DAPs results at enrollment with those from the CHAMACOS (Bradman et al. 2005; Castorina et al. 2010) and NHANES (CDC 2008) pregnancy cohorts. In our study population, the enrollment geometric means for all three summary DAP measures were higher than the NHANES prenatal sample, but lower than CHAMACOS prenatal 1 sample.
Fig 1.
Prenatal comparisons of sum DAPs between Ecuador. CHAMACOS. NHANES pregnancy cohorts, Ecuador and CHAMACOS 1 prenatal samples were collected at approximately 18 and 13 weeks gestation, respectively. XHAXES prenatal samples were collected at approximately 24 weeks gestation. References: NHANES 2001–2002 (Centers for Disease Control and Prevention, 2008); CHAMACOS 1999–2000 (Bradman et al., 2005)
Based on the regression modeling (Table 4a), we found evidence of a decrease in Total DAP levels from enrollment to delivery (p = 0.01). However, we did not find any statistically significant trends in Total DAP levels over pregnancy (p=0.14), but note that, descriptively, the Total DAP decreased on average.
Table 4.
Changes in urinary pesticide metabolite concentrations, by various comparison time points,
| Prenatal change (20 vs. 34 weeks) (n=116) |
Enrollment vs. Delivery (n=50)b |
Rose worker vs. Other (prenatal, n=116) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Pesticide Metabolite |
Estimate | 95% CI | P | Estimate | 95% CI | P | Estimate | 95% CI | P |
| a. | |||||||||
| Total DAP | 0.73 | 0.48, 1.11 | 0.14 | 0.52 | 0.32, 0.86 | 0.01 | 1.39 | 0.86, 2.23 | 0.18 |
| Total DM | 0.77 | 0.50, 1.18 | 0.23 | 0.68 | 0.39, 1.18 | 0.17 | 1.25 | 0.77, 2.02 | 0.37 |
| DMTP | 0.71 | 0.41, 1.23 | 0.22 | 0.54 | 0.24, 1.21 | 0.13 | 1.20 | 0.69, 2.10 | 0.51 |
| b. | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| TCPy | 0.81 | 0.48, 1.38 | 0.44 | 0.27 | 0.14, 0.50 | <0.001 | 1.32 | 0.74, 2.33 | 0.35 |
| PTU | 1.55 | 0.96, 2.51 | 0.07 | 1.34 | 0.75, 2.41 | 0.33 | 1.25 | 0.73, 2.16 | 0.42 |
| ETU | 1.14 | 0.79, 1.66 | 0.49 | 1.05 | 0.57, 1.95 | 0.87 | 0.98 | 0.65, 1.48 | 0.92 |
| Cayambe-Pedro Moncayo region of Ecuador, 2010–2012a | |||||||||
Changes are multiplicative effects on the geometric mean pesticide levels for the change in predictor (unadjusted).
One woman was lost to follow-up at delivery so she was excluded in the modeling, resulting in an enrollment sample n = 25 and delivery sample n = 25.
Table 3a presents DAP summary statistics by current occupation across the n=116 prenatal samples. Based on the regression modeling (Table 4a), we did not find evidence of any differences in DAP levels between rose-workers and others (p=0.18), though estimated DAP levels were higher in rose-workers.
Table 3.
Summary of urinary metabolite concentrations, by occupational group, Cayambe-Pedro Moncayo region of Ecuador, 2010–2012
| 3a. | ||||||
|---|---|---|---|---|---|---|
| DAP metabolites | Samplea | DF | Geo.Mean | 50th | 90th | Max |
| Total DAP (nmol/L) | RW | -- | 82.34 | 85.98 | 566.59 | 778.52 |
| Other | -- | 59.13 | 62.49 | 169.43 | 702.23 | |
| Total DM (nmol/L) | RW | -- | 46.48 | 45.37 | 198.28 | 616.91 |
| Other | -- | 37.52 | 36.53 | 120.61 | 615.19 | |
| DMP (ng/mL) | RW | 0.32 | 7.25 | 2.83 | 57.00 | 172.88 |
| Other | 0.38 | 7.33 | 2.83 | 37.87 | 167 57 | |
| DMTP (ng/mL) | RW | 0.86 | 27.75 | 34.30 | 189.06 | 371.79 |
| Other | 0.90 | 22.93 | 28.03 | 62.51 | 258.97 | |
| DMDTP (ng/mL) | RW | 0.14 | 0.90 | 0.45 | 34.63 | 187.77 |
| Other | 0.08 | 0.65 | 0.45 | 0.45 | 188.65 | |
| Total DE (nmol/L) | RW | -- | 8.29 | 1.71 | 158.69 | 773.67 |
| Other | -- | 5.14 | 1.71 | 75.57 | 671.11 | |
| DEP (ng/mL) | RW | 0.41 | 5.03 | 0.92 | 80.84 | 306.20 |
| Other | 0.36 | 3.75 | 0.92 | 58.37 | 287.68 | |
| DETP (ng/mL) | RW | 0.32 | 1.55 | 0.42 | 42.80 | 249.49 |
| Other | 0.28 | 1.21 | 0.42 | 16.82 | 199.64 | |
| DEDTP (ng/mL) | RW | 0.11 | 0.69 | 0.38 | 35.05 | 217.98 |
| Other | 0.08 | 0.55 | 0.38 | 0.38 | 183.78 | |
| 2b. | ||||||
|---|---|---|---|---|---|---|
| Other metabolites (ng/mL) |
||||||
| TCPy | RW | 0.86 | 0.94 | 1.04 | 4.64 | 10.99 |
| Other | 0.80 | 0.73 | 0.89 | 3.06 | 7.86 | |
| DCCA | RW | 0.06 | 0.10 | 0.07 | 0.07 | 16.59 |
| Other | 0.08 | 0.10 | 0.07 | 0.07 | 9.80 | |
| 3PBA | RW | 0.35 | 0.12 | 0.07 | 0.46 | 3.93 |
| Other | 0.34 | 0.12 | 0.07 | 0.43 | 1.43 | |
| PTU | RW | 0.85 | 0.81 | 1.02 | 2.81 | 4.27 |
| Other | 0.84 | 0.65 | 0.73 | 3.76 | 6.30 | |
| ETU | RW | 1.00 | 3.96 | 3.61 | 10.41 | 131.77 |
| Other | 0.96 | 3.95 | 5.24 | 14.20 | 23.09 | |
Abbreviations: OP, organophosphate; DF, detection frequency; Max, maximum; RW, rose worker.
There were a total of 66 samples for rose workers and 50 samples for the others (n=116); Rose Workers were currently employed in rose industry at the time of urine sample collection; Others were currently employed in other non-agricultural industries or not currently working at the time of urine sample collection.
Limits of detection for DAP metabolites reported in Table 2.
Limits of detection for Other metabolites reported in Table 2.
After adjusting for weeks pregnant and current employment in the rose industry, we did not observe strong evidence of within-woman correlation in any of the DAP measures (DMTP ICC 0.0, p=1.0; total DMP ICC 0.09, p=0.34; Total DAP ICC 0.28, p=0.10).
3.3 Other Metabolites
Table 2b presents the summary statistics for five specific pesticide metabolites measured in our population - TCPy, DCCA, 3PBA, PTU, and ETU, by enrollment/delivery time point. Detection frequencies were the highest for the ETU and PTU urinary metabolite concentrations. We found 100% detection frequency for ETU at enrollment and 96% at delivery. Our prenatal median ETU concentration level was 3.8 ug/L, reaching 10.41 ug/L at the 90th percentile. We found over 80% detection frequency for PTU at enrollment and delivery. We also found 100% detection frequency for TCPy at enrollment and 60% at delivery. The metabolite MDA was also analyzed; however, MDA was not detected for any individual at enrollment and was detected for 2 individuals at delivery. Overall MDA was only detected 6.8% of the time (results not shown).
Table 3b presents the summary statistics for the five specific metabolites by current employment status across prenatal samples only. Based on the regression modeling (Table 4b), we found evidence of a decrease in TCPy from enrollment to delivery (p <0.001). However, we did not find evidence that TCPy levels decreased over pregnancy. We did not find evidence of a change in ETU or PTU levels at delivery (in fact, estimated means were higher at delivery), even though this pesticide was observed at high levels in the study cohort. We found marginal evidence that PTU actually increased over pregnancy (p =0.07). We did not find evidence of any differences in pesticide levels by current employment status (rose worker versus other). In fact, during pregnancy, estimated geometric mean ETU metabolite concentrations were slightly lower among women currently working in the rose industry.
After adjusting for weeks pregnant and current employment in the rose industry, we observed some within-woman correlation in TCPy levels (ICC 0.25, p=0.01) and in PTU levels (ICC 0.24, p=0.03), but no evidence of within-woman correlation in ETU levels (ICC 0.0, p=1.0).
3.4 Assessment of presence of chlorpyrifos
Across all samples (n=141), we observe high correlations between TCPy and DETP (rho = 0.77, p < 0.001); between TCPy and DEP (rho = 0.84, p < 0.001); and between DETP and DEP (rho = 0.92, p < 0.001). Restricting to only the participants with levels of all three pesticides above the limit of detection, we observed even stronger correlations, specifically the correlation between TCPy and DETP is rho = 0.99 (p < 0.001), between TCPy and DEP is rho = 1.00 (p < .001), and between DETP and DEP is rho = 0.99 (p < 0.001).
4. Discussion
In this paper, we present the first findings regarding prenatal pesticide exposure levels in pregnant workers in Ecuador. We present the results of our analysis quantifying levels of 12 specific metabolites associated with the organophosphate, bis-dithiocarbamate, and pyrethroid classes of pesticides among 26 pregnant working women in an agricultural region of Ecuador participating in a pilot birth cohort study. Our study is the first of its kind to quantify prenatal pesticide exposure levels in working women in Ecuador and one of few worldwide, particularly in the developing world and in female populations working in corporate export-led agricultural industry.
While it is important to note that these are exploratory analyses and should be interpreted with caution due to the small sample size and non-representative convenience sample, there are several notable findings from this pilot study. Most notably, we found elevated levels of the ETU urinary metabolite in our entire sample (100% detection frequency at enrollment and 96% post-delivery). The ethylenebisdithiocarbamates, or EBDCs, are commonly used fungicides in agricultural production in Ecuador (Cole et al. 1997; Orozco et al. 2009). EBDCs are metabolized into a more toxic and carcinogenic compound, ETU, which has also been associated with decreased thyroid hormone levels and thyroid gland disorders (Houeto et al. 1995; Panganiban et al. 2004). The levels reported in our study population were much higher than what has been reported for the CHAMACOS pregnancy cohort population (Castorina et al. 2010) - a comparable agricultural community - and for a representative U.S. population of pregnant women (CDC 2013b). Unlike our findings where ETU detection frequencies were very high (>95%) and levels were consistently above the LOD, both of the latter studies report low detection frequencies and median levels of ETU below the limit of detection. Our detectable levels are comparable to recently published findings in a pregnant agricultural population in Costa Rica (Wendel de Joode van et al. 2014).
It is possible that the high levels of ETU found in the sample may be explained by contaminated food sources. In Ecuador, the use of EBDC fungicides, and in particular the use of mancozeb, is common in the production of potatoes (Kromann et al. 2011; Orozco et al. 2007; Schutz 2014). Potato consumption is high in Ecuador, especially in the mountain regions, such as the Pichincha Province, where our study was conducted. Ingesting food treated with EBDC fungicides may be a potential exposure route (CDC 2013a). Research suggests that heating food that is treated with EBDC will result in higher exposure to ETU, thus highlighting another important exposure pathway (Lentza-Rizos 1990). Because we found high levels in our entire sample, not just the rose workers, future research in this region should investigate contaminated food consumption as a potential pathway of exposure in these communities.
Exposure to EBDC fungicides may also occur through water. While EBDC fungicides are highly insoluble and break down quickly in the environment, ETU is highly water soluble. Studies of environmental fate of common use fungicides in the U.S. have found contamination in surface and groundwater (Smalling et al. 2013; Reilly et al. 2012); however few have documented ETU contamination (CDC 2013a). In developing countries and in specifically in Ecuador where use of EBDC fungicides, namely mancozeb, is common and widespread, there are no studies to our knowledge that have analyzed the water sources for ETU contamination in agricultural communities. Future studies should incorporate methods to assess community water sources in the region as another potential exposure pathway.
Additionally, we observed high detection frequencies for five of the six OP metabolites and for two of the other pesticide metabolites (PTU, TCPy). Further, in our sample, the dimethyl OP metabolite concentrations (total DM) were consistently higher than the diethyl (total DE) metabolite concentrations, which is consistent with previous research in other populations (Barr et al. 2004; Bradman et al. 2005; CDC 2001; Fenske et al. 2000). Previous research indicates that the difference between the DM and DE metabolite concentration levels may be due to a longer half-life for dimethyl OP pesticides (Bradman et al. 2005). Additionally, differences in home or community pesticide exposure, including environmental and food-related exposure pathways, may also explain this difference. These differences should be further explored in a larger future investigation.
Detection frequencies were high for TCPy and our results indicate a high correlation between TCPy, DEP, and DETP. This result is indicative of the presence of chlorpyrifos. Studies on pesticides use in agricultural production in the northern highland region of Ecuador have reported moderate use of chlorpyrifos, but not at the same magnitude as the use of fungicides such as mancozeb (Orozco et al. 2009; Schutz 2014).
We found no evidence of statistical differences in pesticide metabolite levels by current employment status, though we observed the highest pesticide levels among rose workers and more variability in concentration levels among rose workers. As acknowledged above, these findings may be partially explained by the limited sample size of this pilot study and by the use of a non-random sample. Recognizing these limitations, there may be several additional explanations for why current rose workers did not have significantly higher levels compared to their non-agricultural working counterparts.
In our study, we consider urinary DAP metabolite concentration levels as an indicator of OP pesticide exposure. The literature suggests, however, that urinary metabolite levels during pregnancy may reflect the exposure of the mother to both the parent compounds and to environmental degradation products (Lu et al. 2005; Quiros-Alcala et al. 2012). It may be that our findings are reflecting environmental exposure, not just occupational exposure. Our study population (rose workers and other workers) resided in the valley region where most flower farms are located. It is possible that exposure to these pesticides and their environmental degradation products may occur via water sources in the community, or by air. Additionally, it is not uncommon that these classes of pesticides are applied on other food crops, which are readily available in the region for consumption. Future research using a larger sample should address these additional potential pathways of pesticide exposure in the region.
Alternatively, using urinary DAP metabolite concentrations as the primary indicator of OP pesticide exposure may not represent the total exposure of our rose worker population. Certain OPs do not devolve into urinary DAP metabolites. A recent study of flower producing greenhouses in Brazil found a high percentage of use of the OPs methamidophos and acephate in the flower production process; both of which are OPs that do not devolve into urinary DAPs (Ribeiro et al. 2012). The use of urinary DAP metabolite measurement may not fully capture OP exposure levels in our population if the Ecuadorian flower industry relies on the heavy use of these non-devolving OP pesticides. Future studies should specifically assess and determine which pesticides are most often used in the Ecuadorian flower production process to address this potential issue. Furthermore, there is a need for future research to characterize all environmental sources of exposure as well as determine if certain pesticides have gone undetected in rose workers.
In general, detection frequencies and geometric means for the six DAP urinary metabolites were lower at the post-delivery interview than enrollment, but we did not find statistically significant evidence of temporal trends in pesticide exposure levels over the course of pregnancy. This general temporal trend, however, is in the direction we would expect; as these women progress through their pregnancy they may change their job tasks and/or start their maternity leave in later pregnancy. In our study, 81% of women reported working after 30 weeks of pregnancy.
When we conducted a longitudinal analysis of our urine measures to assess intra-woman variability, we found that the correlation between within-woman pesticide levels over pregnancy was weak, indicating within-woman variability of pesticide metabolites levels across pregnancy. How pesticide levels vary over the entire pregnancy within an individual is not well understood. In the literature, longitudinal studies of prenatal pesticide exposure typically collect, on average, 1–3 urine samples per woman over pregnancy, often due to logistical and cost constraints (Engel et al. 2007; Eskenazi et al. 2004; Kongtip et al. 2014; Rauch et al. 2012; Ye et al. 2009). There are challenges, however, in using urinary metabolite levels to estimate non-persistent pesticide exposure levels during pregnancy. Estimating the internal dose of exposure from biospecimens may not reflect chronic exposure over the course of pregnancy due to the short half-life of these non-persistent pesticides (Wessels et al. 2003). Our finding emphasizes the need to further examine variability across pregnancy to better understand how to define “exposed” versus “unexposed” with a highly temporally varying exposure in order to accurately characterize prenatal exposure patterns in a population. This is especially important for agricultural workers, particularly flower workers, who tend to change their tasks fairly often and especially during pregnancy. Future studies should incorporate multiple measures throughout pregnancy to assess patterns of variation in order to more accurately estimate prenatal exposure. Moreover, by collecting multiple samples over pregnancy, it may be possible to conduct statistical power analyses evaluating how many samples are necessary, and at which time points in pregnancy, to appropriately classify an individual as “exposed” based on levels at which health effects are expected to arise.
This pilot study has several limitations. We must be cautious in the interpretation of our findings, as a small sample size limited our statistical analysis. Furthermore, we did not randomly sample participants, but enrolled all eligible women that we could identify, resulting in a convenience sample that may not be a representative sample of all working women in the region, thus compromising generalizability of our results. Specifically, our sample may not represent the true pesticide exposure distribution for all rose workers in the region. During our recruitment period, the study team was made aware of women who expressed interest in participating in this pilot study, but who were scared because of work place intimidation and hostile work conditions. This selection bias may have resulted in excluding those women who may have a higher pesticide exposure due to poorer working conditions. Additionally, enrollment in our study did not occur at the exact same gestational age time point for all participants. As a pilot study, we were assessing the best recruitment and retention approaches so our inclusion criterion was broader to include women in late 1st trimester and early 2nd trimester. Future studies should include a more narrow gestational age inclusion criterion. Finally, some of the biomarkers measured could be derived from exposure to the preformed metabolite in the environment.
Despite these limitations, this study has several important strengths. To our knowledge, this is the first study of its kind in Ecuador that has quantified pesticide exposure levels among women working in or residing near large-scale cut-flower farms, with a specific focus on prenatal levels. This study was longitudinal in design and, included, on average, a total of 5.4 urine samples per woman throughout the 1st, 2nd and 3rd trimesters. Additionally, urine samples in our study population were always taken as a first morning void. The literature suggests variability in same-day urine samples, depending on the time of day that the collection occurred (Bradman et al. 2013). The first morning void may more accurately reflect the total metabolite concentration levels compared to samples collected at other times during the day, as it reflects an extended period of accumulation (Kissel et al. 2005). Finally, this pilot study used a community-based participatory approach building on years of collaboration, which allowed us to retain the majority of our study population throughout the study period. We had no losses-to-follow-up during pregnancy and only one woman was lost after delivery.
5. Conclusion
In this study of 26 working women residing in a highland region of Ecuador, we observed high detection frequencies for several OP and bis-dithiocarbamate metabolites. Among the pesticides tested in this population, concentrations were the highest for the ETU metabolite and were elevated in our entire sample, indicating potential environmental exposure pathways that should be further investigated in a larger study. Our findings, while limited by the small sample size and use of a convenience sample, provide an initial characterization of prenatal pesticide exposure levels and how these levels vary over pregnancy. Furthermore, these pilot data set the stage for a larger, more comprehensive longitudinal cohort study to assess the impact of prenatal pesticide exposure on infant and child development.
Acknowledgements
We thank our consortium partners in Ecuador: Dr. William Waters, director of the Research Institute for Health and Nutrition-Universidad de San Francisco de Quito (USFQ), and the USFQ Center for Technology Development and Transfer (USFQ-CTT). We thank our study team including our study nurse, Katty Turqueres, and field coordinator, Luis Pena. We also thank the staff at the Casa Campesina Cayambe, the members of the Community Advisory Board, the local community leaders and local hospital and clinic staff, and, most of all, the study participants. Dr. Handal was supported by a National Institute of Environmental Health Sciences (NIEHS) Grant #5R21ES19285-2 and faculty field research grants from the University of New Mexico Latin American and Iberian Institute (LAII). This project was also supported in part by the Dedicated Health Research Funds from the University of New Mexico School of Medicine.
Footnotes
Conflict of Interest The authors declare that they have no conflict of interest.
References
- Alavanja MC. Introduction: pesticides use and exposure extensive worldwide. Reviews on Environmental Health. 2009;24:303–309. doi: 10.1515/reveh.2009.24.4.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr DB, et al. Strategies for biological monitoring of exposure for contemporary-use pesticides Toxicology and Industrial Health. 1999;15:168–179. doi: 10.1191/074823399678846556. [DOI] [PubMed] [Google Scholar]
- Barr DB, et al. Concentrations of dialkyl phosphate metabolites of organophosphorus pesticides in the U.S. population. Environmental Health Perspectives. 2004;112:186–200. doi: 10.1289/ehp.6503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman T, Hochner-Celnikier D, Barr D, Needham L, Amitai Y, Wormser U, Richter E. Pesticide exposure among pregnant women in Jerusalem, Israel: Results of a pilot study. Environment International. 2011;37:198–203. doi: 10.1016/j.envint.2010.09.002. [DOI] [PubMed] [Google Scholar]
- Bradman A, et al. Organophosphate urinary metabolite levels during pregnancy and after delivery in women living in an agricultural community. Environmental Health Perspectives. 2005;113:1802–1807. doi: 10.1289/ehp.7894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradman A, et al. Variability of organophosphorous pesticide metabolite levels in spot and 24-hr urine samples collected from young children during 1 week. Environmental Health Perspectives. 2013;21:118–124. doi: 10.1289/ehp.1104808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breilh J. [New model of accumulation and agro-business: the ecological and epidemiological implications of the Ecuadorian cut flower production] Ciencia & Saude Coletiva. 2007;12:91–104. doi: 10.1590/s1413-81232007000100013. [DOI] [PubMed] [Google Scholar]
- Breilh J, et al. CEAS (ed) Latin American Health Watch: Alternative Latin American Health Report. Quito, Ecuador: Global Health Watch; 2005. Floriculture and the health divide: a struggle for fair and ecological flowers. [Google Scholar]
- Castorina R, et al. Comparison of current-use pesticide and other toxicant urinary metabolite levels among pregnant women in the CHAMACOS cohort and NHANES. Environ Health Perspect. 2010;118:856–863. doi: 10.1289/ehp.0901568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC. National Report on Human Exposure to Environmental Chemicals. Centers for Disease Control and Prevention. Atlanta, GA: National Center Environmental Health; 2001. [Google Scholar]
- CDC. Second National Report on Human Exposure to Environmental Chemicals. Centers for Disease Control and Prevention. Atlanta, GA: National Center Environmental Health; 2003. [Google Scholar]
- National Health and Nutrition Examination Survey Data. U.S. Department of Health and Human Services. Centers for Disease Control and Prevention; 2008. [Accessed 25 May 2014]. http://www.cdc.gov.libproxy.unm.edu/nchs/about/major/nhanes/datalink.htm. [Google Scholar]
- CDC. Biomonitoring Summary: Ethylenethiourea Propylenethiourea. Atlanta, GA: Centers for Disease Control and Prevention; 2013a. [Google Scholar]
- CDC. Fourth National Report on Human Exposure to Environmental Chemicals. Atlanta, GA: Centers for Disease Control and Prevention; 2013b. [Google Scholar]
- Cole DC, Carpio F, Julian J, Leon N, Carbotte R, De Almeida H. Neurobehavioral outcomes among farm and nonfarm rural Ecuadorians. Neurotoxicol Teratol. 1997;19:277–286. doi: 10.1016/s0892-0362(97)00019-6. [DOI] [PubMed] [Google Scholar]
- Colosio C, et al. [Occupational exposure to fungicides in floriculture in Ecuador] Giornale Italiano di Medicina del Lavoro ed Ergonomia. 2003;25(Suppl):107–108. [PubMed] [Google Scholar]
- Direccion de Inteligencia Comercial e Inversiones. Analisis Sectorial de Flores ProEcuador. Instituto de Promocion de Exportaciones e Inversiones. Quito, Ecuador: Ministerio de Relaciones Exteriores Comercio e Integracion; 2011. Available at http://www.proecuador.gob.ec/wp-content/uploads/downloads/2012/01/PROEC-AS2011-FLORES.pdf. [Google Scholar]
- Ecobichon D. Pesticide use in developing countries. Toxicology. 2001;160(1–3):6. doi: 10.1016/s0300-483x(00)00452-2. [DOI] [PubMed] [Google Scholar]
- Engel SM, et al. Prenatal organophosphate metabolite and organochlorine levels and performance on the Brazelton Neonatal Behavioral Assessment Scale in a multiethnic pregnancy cohort. American Journal of Epidemiology. 2007;165:1397–1404. doi: 10.1093/aje/kwm029. [DOI] [PubMed] [Google Scholar]
- Eskenazi B, et al. Association of in utero organophosphate pesticide exposure and fetal growth and length of gestation in an agricultural population. Environmental Health Perspectives. 2004;112:1116–1124. doi: 10.1289/ehp.6789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskenazi B, et al. Organophosphate pesticide exposure and neurodevelopment in young Mexican-American children. Environmental Health Perspectives. 2007;115:792–798. doi: 10.1289/ehp.9828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskenazi B, et al. Pesticide toxicity and the developing brain. Basic & Clinical Pharmacology & Toxicology. 2008;102:228–236. doi: 10.1111/j.1742-7843.2007.00171.x. [DOI] [PubMed] [Google Scholar]
- Fenske RA, Kissel JC, Lu C, Kalman DA, Simcox NJ, Allen EH, Keifer MC. Biologically based pesticide dose estimates for children in an agricultural community. Environmental Health Perspectives. 2000;108:515–520. doi: 10.1289/ehp.00108515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandjean P, Landrigan PJ. Neurobehavioural effects of developmental toxicity. Lancet Neurol. 2014;13:330–338. doi: 10.1016/S1474-4422(13)70278-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallam D, Liu P, Lavers G, Pikauskas P, Rapsomanikis G, Claro J. The Market for Non-Traditional Agricultural Exports. Raw Material, Tropical and Horticultural Products Service Commodities and Trade Division. 2004 [Google Scholar]
- Handal AJ, Harlow SD, Breilh J, Lozoff B. Occupational Exposure to Pesticides During Pregnancy and Neurobehavioral Development of Infants and Toddlers. Epidemiology. 2008;19:851–859. doi: 10.1097/EDE.0b013e318187cc5d. [DOI] [PubMed] [Google Scholar]
- Handal AJ, Lozoff B, Breilh J, Harlow SD. Effect of community of residence on neurobehavioral development in infants and young children in a flower-growing region of Ecuador. Environmental Health Perspectives. 2007a;115:128–133. doi: 10.1289/ehp.9261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handal AJ, Lozoff B, Breilh J, Harlow SD. Neurobehavioral development in children with potential exposure to pesticides. Epidemiology. 2007b;18:312–320. doi: 10.1097/01.ede.0000259983.55716.bb. [DOI] [PubMed] [Google Scholar]
- Handal AJ, Lozoff B, Breilh J, Harlow SD. Sociodemographic and nutritional correlates of neurobehavioral development: a study of young children in a rural region of Ecuador. Pan American Journal of Public Health. 2007c;21:292–300. doi: 10.1590/s1020-49892007000400004. [DOI] [PubMed] [Google Scholar]
- Handal AJ, McGough-Maduena A, Paez M, Skipper B, Rowland AS, Fenske RA, Harlow SD. A pilot study comparing observational and questionnaire surrogate measures of pesticide exposure among residents impacted by the Ecuadorian flower industry. Archives of environmental & occupational health. 2014 doi: 10.1080/19338244.2013.879563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harari R, et al. Seguridad, Salud, y Ambiente en la Floricultura. Quito, Ecuador: IFA-Promesa; 2004. [Google Scholar]
- Houeto P, Bindoula G, Hoffman JR. Ethylenebisdithiocarbamates and ethylenethiourea -possible human health-hazards. Environmental Health Perspectives. 1995;103:568–573. doi: 10.1289/ehp.95103568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huen K, Bradman A, Harley K, Yousefi P, Barr DB, Eskenazi B, Holland N. Organophosphate pesticide levels in blood and urine of women and newborns living in an agricultural community. Environmental Research. 2012;117:8–16. doi: 10.1016/j.envres.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurewicz J, Hanke W. Prenatal and childhood exposure to pesticides and neurobehavioral development: Review of epidemiological studies. International Journal of Occupational Medicine and Environmental Health. 2008;21:121–132. doi: 10.2478/v10001-008-0014-z. [DOI] [PubMed] [Google Scholar]
- Kissel JC, et al. Comparison of organophosphorus pesticide metabolite levels in single and multiple daily urine samples collected from preschool children in Washington State. Journal of Exposure Analysis and Environmental Epidemiology. 2005;15:164–171. doi: 10.1038/sj.jea.7500384. [DOI] [PubMed] [Google Scholar]
- Kongtip P, Nankongnab N, Woskie S, Phamonphon A, Tharnpoophasiam P, Wilaiwan K, Srasom P. Organophosphate urinary metabolite levels during pregnancy, delivery and postpartum in women living in agricultural areas in Thailand. Journal of Occupational Health. 2014;55:367–375. doi: 10.1539/joh.13-0040-oa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kromann P, Pradel W, Cole DC, Taipe A, Forbes GA. Use of the Environmental Impact Quotient to Estimate Health and Environmental Impacts of Pesticide Usage in Peruvian and Ecuadorian Potato Production. Journal of Environmental Protection. 2011:581–591. [Google Scholar]
- Lentza-Rizos C. Ethylenethiourea (ETU) in relation to use of ethylenebisdithiocarbamate (EBDC) fungicides. Reviews of Environmental Contamination and Toxicology. 1990;115:1–37. doi: 10.1007/978-1-4612-3416-6_1. [DOI] [PubMed] [Google Scholar]
- Llop S, et al. Prenatal and postnatal insecticide use and infant neuropsychological development in a multicenter birth cohort study. Environment International. 2013;59:175–182. doi: 10.1016/j.envint.2013.06.010. [DOI] [PubMed] [Google Scholar]
- London L, de GS, Wesseling C, Kisting S, Rother HA, Mergler D. Pesticide usage and health consequences for women in developing countries: out of sight, out of mind? International Journal of Occupational and Environmental Health. 2002;8:46–59. doi: 10.1179/oeh.2002.8.1.46. [DOI] [PubMed] [Google Scholar]
- Lu C, Bravo R, Caltabiano LM, Irish RM, Weerasekera G, Barr DB. The presence of dialkylphosphates in fresh fruit juices: implication for organophosphorus pesticide exposure and risk assessments. Journal of Toxicology and Environmental Health Part A. 2005;68:209–227. doi: 10.1080/15287390590890554. [DOI] [PubMed] [Google Scholar]
- Lubin JH, et al. Epidemiologic evaluation of measurement data in the presence of detection limits. Environmental Health Perspectives. 2004;112:1691–1696. doi: 10.1289/ehp.7199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montesano MA, Olsson AO, Kuklenyik P, Needham LL, Bradman AS, Barr DB. Method for determination of acephate, methamidophos, omethoate, dimethoate, ethylenethiourea and propylenethiourea in human urine using high-performance liquid chromatography-atmospheric pressure chemical ionization tandem mass spectrometry. J Expo Sci Environ Epidemiol. 2007;17:321–330. doi: 10.1038/sj.jes.7500550. [DOI] [PubMed] [Google Scholar]
- Olsson AO, et al. A liquid chromatography--tandem mass spectrometry multiresidue method for quantification of specific metabolites of organophosphorus pesticides, synthetic pyrethroids, selected herbicides, and deet in human urine. Anal Chem. 2004;76:2453–2461. doi: 10.1021/ac0355404. [DOI] [PubMed] [Google Scholar]
- Orozco F, Cole DC, Munoz V, Altamirano A, Wanigaratne S, Espinosa P, Munoz F. Relationships among production systems, preschool nutritional status, and pesticide-related toxicity in seven ecuadorian communities: a multi-case study approach. Food and Nutrition Bulletin. 2007;28:S247–S257. doi: 10.1177/15648265070282S204. [DOI] [PubMed] [Google Scholar]
- Orozco FA, Cole DC, Forbes G, Kroschel J, Wanigaratne S, Arica D. Monitoring adherence to the international code of conduct: highly hazardous pesticides in central Andean agriculture and farmers' rights to health. International Journal of Occupational and Environmental Health. 2009;15:255–268. doi: 10.1179/oeh.2009.15.3.255. [DOI] [PubMed] [Google Scholar]
- Panganiban L, Cortes-Maramba N, Dioquino C, Suplido ML, Ho H, Francisco-Rivera A, Manglicmot-Yabes A. Correlation between blood ethylenethiourea and thyroid gland disorders among banana plantation workers in the Philippines. Environmental Health Perspectives. 2004;112:42–45. doi: 10.1289/ehp.6499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prapamontol T, et al. Cross validation of gas chromatography-flame photometric detection and gas chromatography-mass spectrometry methods for measuring dialkylphosphate metabolites of organophosphate pesticides in human urine. Int J Hyg Environ Health. 2014;217:554–566. doi: 10.1016/j.ijheh.2013.10.005. [DOI] [PubMed] [Google Scholar]
- Quiros-Alcala L, et al. Organophosphorous pesticide breakdown products in house dust and children's urine. Journal of Exposure Science & Environmental Epidemiology. 2012;22:559–568. doi: 10.1038/jes.2012.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch SA, et al. Associations of prenatal exposure to organophosphate pesticide metabolites with gestational age and birth weight. Environmental Health Perspectives. 2012;120:1055–1060. doi: 10.1289/ehp.1104615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauh VA, et al. Impact of prenatal chlorpyrifos exposure on neurodevelopment in the first 3 years of life among inner-city children. Pediatrics. 2006;118:E1845–E1859. doi: 10.1542/peds.2006-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly TJ, Smalling KL, Orlando JL, Kuivila KM. Occurrence of boscalid and other Selected fungicides in surface water and groundwater in three targeted use areas in the United States. Chemosphere. 2012 Sep;89(3):228–234. doi: 10.1016/j.chemosphere.2012.04.023. PubMed PMID: 22564453. [DOI] [PubMed] [Google Scholar]
- Ribeiro MG, Colasso CG, Monteiro PP, Pedreira Filho WR, Yonamine M. Occupational safety and health practices among flower greenhouses workers from Alto Tiete region (Brazil) The Science of the Total Environment. 2012;416:121–126. doi: 10.1016/j.scitotenv.2011.11.002. [DOI] [PubMed] [Google Scholar]
- Rice D, Barone S. Critical periods of vulnerability for the developing nervous system: Evidence from humans and animal models. Environmental Health Perspectives. 2000;108:511–533. doi: 10.1289/ehp.00108s3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosas LG, Eskenazi B. Pesticides and child neurodevelopment. Current Opinion in Pediatrics. 2008;20:191–197. doi: 10.1097/MOP.0b013e3282f60a7d. [DOI] [PubMed] [Google Scholar]
- Sawers L. Nontraditional or new traditional exports: Ecuador’s flower boom. Latin American Research Review. 2005;40(3):28. [Google Scholar]
- Scheuplein R, Charnley G, Dourson M. Differential sensitivity of children and adults to chemical toxicity - I. Biological basis. Regulatory Toxicology and Pharmacology. 2002;35:429–447. doi: 10.1006/rtph.2002.1558. [DOI] [PubMed] [Google Scholar]
- Schutz L. Survey of Agricultural Practices and Alternatives to Pesticide Use to Conserve Water Resources in the Mojanda Watershed, Ecuador. Future of Food: Journal of Food, Agriculture and Society. 2014;2:76–92. [Google Scholar]
- Smalling KL, et al. Environmental fate of fungicides and other current-use pesticides in a Central California estuary. Marine Pollution Bulletin. 2013;73:144–153. doi: 10.1016/j.marpolbul.2013.05.028. [DOI] [PubMed] [Google Scholar]
- Tobin J. Estimation of Relationships for Limited Dependent Variables. Econometrica. 1958;26:24–36. [Google Scholar]
- Wendel de Joode van B, et al. Aerial Application of Mancozeb and Urinary Ethylene Thiourea (ETU) Concentrations among Pregnant Women in Costa Rica: The Infants’ Environmental Health S tudy (ISA) Environmental Health Perspectives. 2014 doi: 10.1289/ehp.1307679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessels D, Barr DB, Mendola P. Use of biomarkers to indicate exposure of children to organophosphate pesticides: implications for a longitudinal study of children's environmental health. Environmental Health Perspectives. 2003;111:1939–1946. doi: 10.1289/ehp.6179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyatt RM, et al. Residential pesticide use during pregnancy among a cohort of urban minority women. Environmental Health Perspectives. 2002;110:507–514. doi: 10.1289/ehp.02110507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aksoy MA, Behgin JC, editors. World Bank. Global Agricultural Trade and Developing Countries. World Bank; 2005. [Google Scholar]
- World Health Organization. Public Health Impact of Pesticides Used in Agriculture. Geneva: WHO; 1990. [Google Scholar]
- Ye XB, et al. Levels of metabolites of organophosphate pesticides, phthalates, and bisphenol A in pooled urine specimens from pregnant women participating in the Norwegian Mother and Child Cohort Study (MoBa) International Journal of Hygiene and Environmental Health. 2009;212:481–491. doi: 10.1016/j.ijheh.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, et al. Prenatal exposure to organophosphate pesticides and neurobehavioral development of neonates: a birth cohort study in Shenyang, China. PloS One. 2014;9:e88491. doi: 10.1371/journal.pone.0088491. [DOI] [PMC free article] [PubMed] [Google Scholar]