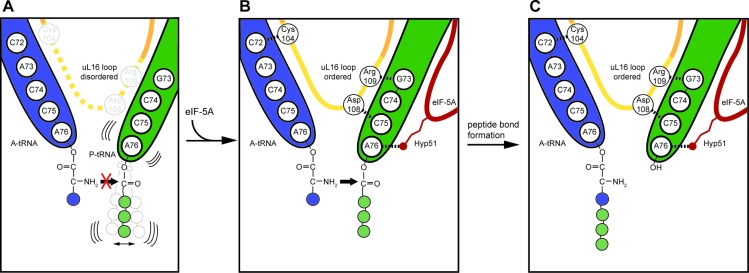
Figure 4.
Model for eIF-5A action on the ribosome. (A) Certain nascent polypeptide chains, such as those containing polyproline stretches, destabilize the P-tRNA (green) and prevent peptide-bond formation with the incoming A-tRNA (blue). (B) The stalled ribosomes are recognized by eIF-5A, which binds such that the modified hypusine 51 (Hyp51) residue interacts with the A76 of the CCA-end of the P-tRNA. This interaction stabilizes the P-tRNA in the optimal geometry for peptide bond formation, leading to ordering of the loop of uL16, which in turn establishes interactions with both A- and P-tRNAs, facilitating efficient peptide bond formation. (C) Peptide bond formation leads to a deacylated tRNA in the P-site and A-tRNA bearing the nascent polypeptide chain extended by one amino acid.