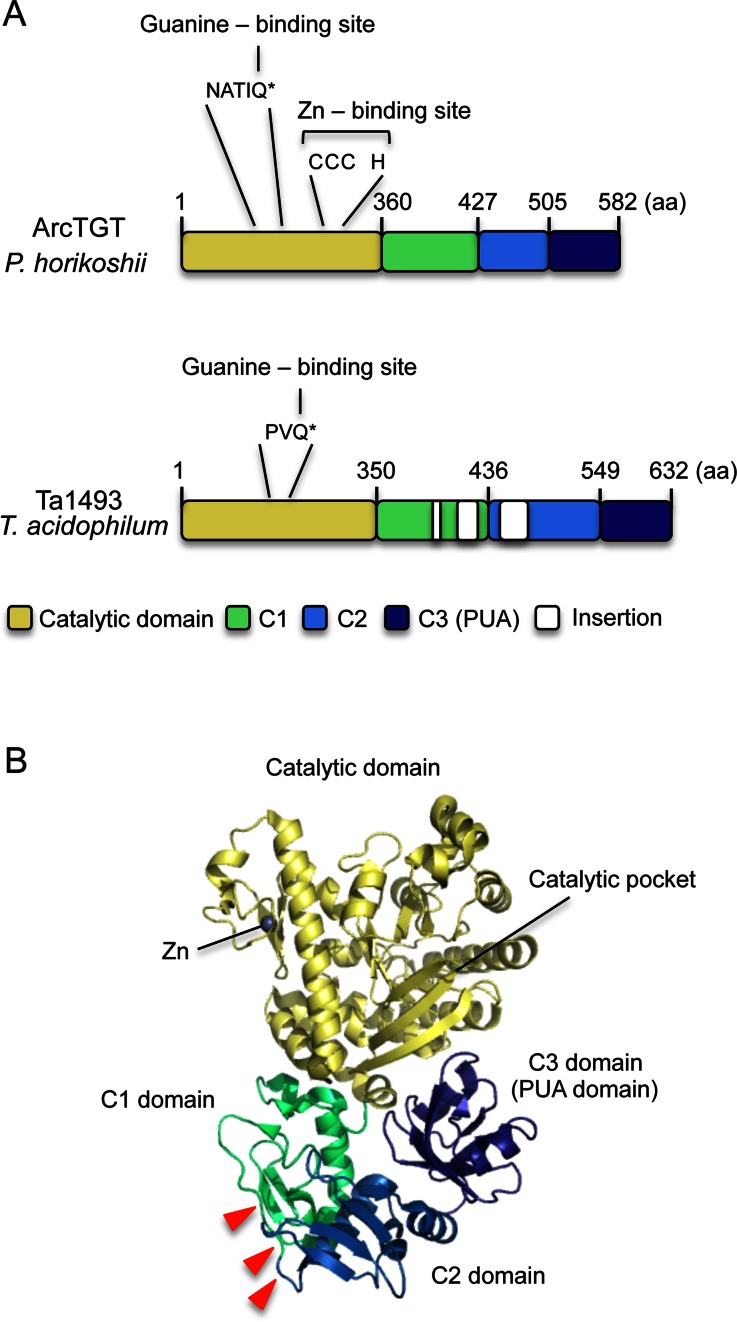
Figure 2.
(A) Comparison of the domain structures of P. horikoshii ArcTGT and T. acidophilum Ta1493 gene product. ArcTGT from P. horikoshii is composed of four domains, catalytic, C1, C2 and C3 (PUA) domains. The guanine- and Zn-binding sites in the catalytic domain are highlighted: Q* in the NATIQ sequence binds the G15 base. In contrast, the Ta1493 gene product possesses three insertions in the C1 and C2 domains and the Zn-binding site (CCCH motif) is missing. In addition, the amino acid sequence around the guanine binding site is different from that in P. horikoshii ArcTGT. (B) Structure of P. horikoshii ArcTGT. Three insertion sites are shown by red triangles.