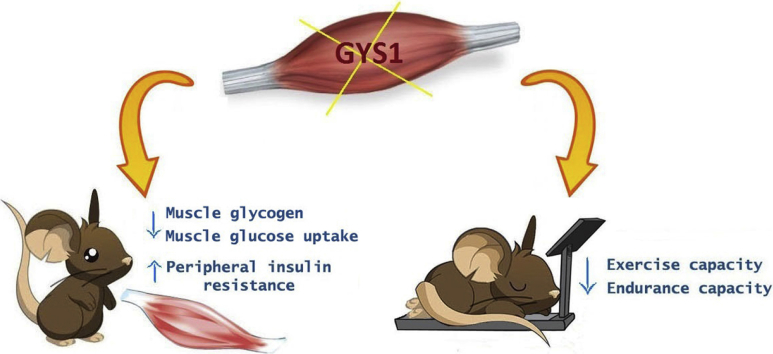
Abstract
Objective
Muscle glucose storage and muscle glycogen synthase (gys1) defects have been associated with insulin resistance. As there are multiple mechanisms for insulin resistance, the specific role of glucose storage defects is not clear. The aim of this study was to examine the effects of muscle-specific gys1 deletion on glucose metabolism and exercise capacity.
Methods
Tamoxifen inducible and muscle specific gys-1 KO mice were generated using the Cre/loxP system. Mice were subjected to glucose tolerance tests, euglycemic/hyperinsulinemic clamps and exercise tests.
Results
gys1-KO mice showed ≥85% reduction in muscle gys1 mRNA and protein concentrations, 70% reduction in muscle glycogen levels, postprandial hyperglycaemia and hyperinsulinaemia and impaired glucose tolerance. Under insulin-stimulated conditions, gys1-KO mice displayed reduced glucose turnover and muscle glucose uptake, indicative of peripheral insulin resistance, as well as increased plasma and muscle lactate levels and reductions in muscle hexokinase II levels. gys1-KO mice also exhibited markedly reduced exercise and endurance capacity.
Conclusions
Thus, muscle-specific gys1 deletion in adult mice results in glucose intolerance due to insulin resistance and reduced muscle glucose uptake as well as impaired exercise and endurance capacity.
In brief
This study demonstrates why the body prioritises muscle glycogen storage over liver glycogen storage despite the critical role of the liver in supplying glucose to the brain in the fasting state and shows that glycogen deficiency results in impaired glucose metabolism and reduced exercise capacity.
Keywords: gys1, Glucose tolerance, Insulin sensitivity, Muscle glucose uptake, Exercise capacity, Inducible muscle-specific knockout (KO) mice
Graphical abstract
Highlights
-
•
Muscle-specific gys1 knockdown in adult mice results in 70% reduction in skeletal muscle glycogen levels.
-
•
Muscle-specific gys1 knockdown leads to glucose intolerance and peripheral insulin resistance.
-
•
Muscle glycogen depletion caused impaired performance, as well as fatigue development during exercise.
1. Introduction
Insulin-stimulated skeletal muscle glucose uptake plays a crucial role in overall glucose metabolism in humans as it accounts for 85% of the total amount of postprandial glucose metabolised [1], [2], [3], [4], [5]. Muscle glycogen synthesis comprises a principal pathway of glucose disposal in both normal individuals and patients with type 2 diabetes (T2D) [6]. T2D is characterised by hyperglycaemia contributed to by peripheral insulin resistance, predominantly within skeletal muscle [1], [2], [7], [8]. Non-oxidative glucose metabolism is primarily affected, with impaired muscle glycogen synthesis considered a principal metabolic defect in insulin resistance [6], [7], [9], [10], [11], [12], [13]. The rate-determining step for muscle glycogen synthesis is catalysed by the enzyme glycogen synthase encoded by gys1.
Previous studies have suggested that skeletal muscle glycogen may be critical for exercise capacity as it is the main energy fuel used in muscle contraction [14]. A strong association has been shown between muscle glycogen depletion, impaired muscle performance and fatigue development during exercise [15], [16]. This association is also supported by findings in patients with Glycogen Storage Disease 0b (GSD0b), characterised by complete elimination of muscle gys1 leading to muscle weakness, pain, cramps and poor exercise performance, with a low maximal workload and death due to cardiac events in childhood [17], [18].
The principal reason for the occurrence of fatigue during exercise is not clear, with potentially many different mechanisms involved. However, it has been strongly suggested that glycogen depletion could be the principal factor leading to fatigue (“glycogen shunt” hypothesis) [16]. Previous findings in humans suggest that glycogen and glycogenolysis are crucial for energy supply during exercise and muscle contraction generally, and that muscle glycogen is required for blood glucose to enter glycolysis [16], [17], [18]. The confounding issue with human studies is that the manipulation of glycogen levels must be undertaken by exercise followed by dietary modification, which themselves can modify insulin action and hence the assessment of glucose metabolism. This is not an issue in patients born with glycogen storage diseases, but, in these individuals, the confounding factor is that the defect is present from conception allowing the possibility of adaptive mechanisms to develop.
Surprisingly, genetically modifying gys1 expression in animals to determine the physiological consequences on glucose homeostasis and exercise capacity has been conducted only thrice. In one study, skeletal muscle gys1 overexpression resulted in increased glycogen synthase activity (10-fold) and glycogen content (up to 5 fold); however, effects on glucose metabolism were not assessed [19]. Whole-body gys1 deletion, examined in another model, led to 90% perinatal mortality due to abnormal cardiac function, showing that gys1 is essential for normal heart development. Counterintuitively, the few surviving mice showed normal heart morphology and function, improved glucose tolerance and normal exercise capacity [20], [21], [22]. Finally, a mutated gys1 that cannot be allosterically activated was “knocked in” in mice, and, despite a 70% reduction in glycogen synthesis and 50% decrease in muscle glycogen content, there was no effect on plasma glucose and insulin levels, glucose tolerance or glucose turnover during a euglycaemic/hyperinsulinaemic clamp [23].
Thus, the results from previous studies have not conveyed a clear mechanism for the role of gys1 on glucose and exercise metabolism. In the study presented herein, we investigated the impact of conditional muscle-specific gys1 deletion on glucose and exercise metabolism in mice.
2. Materials and methods
2.1. Animals
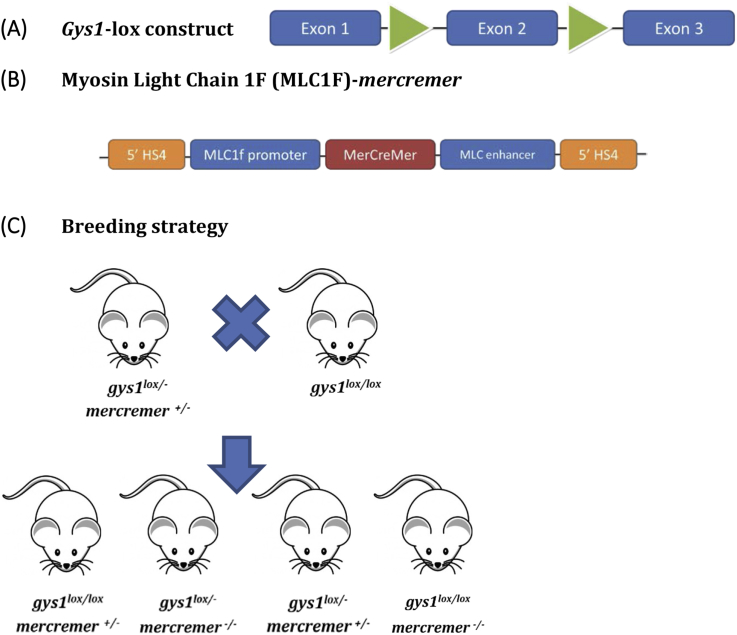
Muscle-specific gys1 KO mice were generated via the conditional Cre-LoxP system. The LoxP targeting construct for gys1 was designed in collaboration with OzGene in WA, Australia (Figure 1A). Exon 2 was selected as the target sequence to be deleted as it contains the UDP-glucose binding site, the excision of which would produce a non-functional protein. Homozygous floxed gys1 mice [gys1lox/lox] were generated on a C57BL/6J background and were mated with the Myosin Light Chain 1F (MLC1F)-mercremer mice (also on a C57BL/6J background), targeting conditional tamoxifen-inducible skeletal muscle-specific gys1 deletion. The MLC1F-mercremer construct (Figure 1B) was generated in order to achieve skeletal muscle-specific gene targeting. Mice hemizygous for gys1Lox and MLC1F-mercremer allele [gys1lox/lox-mercremer+/−] were crossbred with homozygous floxed gys1 mice [gys1lox/lox]. This breeding strategy, outlined in Figure 1C, was used to generate KO mice (gys1(lox/lox) – mercremer(+/−)) and littermate control mice gys1(lox/lox) – mercremer(−/−)] with normal gys1 expression in skeletal muscle. The mice were housed in the BioResources Facility, Austin Health, which had time-controlled artificial lighting with a 12-h dark/light cycle and room temperature of 21.5–23.5 °C. Mice were fed a standard laboratory chow diet ad libitum up to the 10th week of age. During the induction period, tamoxifen (with a concentration of 1 mg/g of food), was incorporated in the standard diet, consisted of 4.8% of energy as fat, 20% of energy as protein and 75.2% of energy as carbohydrate (digestible energy 14 MJ/kg), and was purchased from Specialty Feeds (Glen Forrest, Western Australia). Male mice were placed on tamoxifen diet at 10 weeks of age for 8-weeks, followed by a 4-week tamoxifen-free recovery period on standard chow diet before the beginning of physiological experiments. All animal work was approved by the Austin Health Animal Ethics Committee. For all glucose metabolism investigations, mice were tested as previously described [24].
Figure 1.
(A) Gys1 targeting DNA construct (Ozgene), (B) The final MLC 1F-mercremer DNA transgenic construct. Expression of mercremer is driven by the MLC1F promoter. The MLC enhancer confers the tissue-specific expression. Two fragments of 5′ HS4 insulators were used to improve mercremer expression, (C) Last step of the breeding strategy to generate tamoxifen-inducible gys1 KO mice. gys1lox/lox-mercremer+/− mice were crossed with gys1lox/lox mice. This breeding design can result in 4 different genotypes. The 2 genotypes of interest were selected for experimental (gys1(lox/lox) – mercremer(+/−)) and control (gys1(lox/lox) – mercremer(−/−)) groups respectively. Both groups underwent tamoxifen treatment following their genotype identification.
2.2. RNA extraction and real time PCR
Total RNA was extracted using standard Trizol (Life Technologies) methodology, its concentration determined by spectrophotometry and reverse transcribed using the RT-PCR Introductory System (Promega, Madison, WI). Gene expression was evaluated via real-time PCR analyses using a master mix containing Taqman primer mix, which was made for each target gene and the endogenous reference (Applied Biosystems, Life Technologies, Vic, Australia). For the housekeeping gene, ribosomal RNA was used (18S rRNA 4319413E) (Applied Biosystems, Life Technologies, Vic, Australia). All amplification reactions were performed in triplicate. Results were analysed with Applied Biosystems 7500 system software. The relative quantification method was employed to assess the expression level of the target genes [25].
2.3. Western blotting
Tissues were homogenised in lysis buffer (20 mM Tris, 1 mM EDTA and 0.25 mM sucrose, pH 7.4) and ultracentrifuged to obtain the membrane fractions. The amount of protein in the homogenate was quantified using the Bio-Rad Protein Assay, based on the Bradford method. Equivalent amounts of protein were resolved on an 8–12% SDS-PAGE and transferred onto a PVDF membrane (Immobilon-P transfer membrane, Millipore). Glycogen synthase was detected using the Abcam (EP817Y, ab40810) rabbit monoclonal anti-gys1 primary antibody. GLUT-4 was detected using the Abcam (mAbcam65267) anti-GLUT-4 primary antibody. Hexokinase II was detected using the Abcam (ab76959) mouse monoclonal Anti-Hexokinase II antibody. Glucose-6-phosphate isomerase was detected using the Abcam [1B7D7] (ab66340) mouse monoclonal Anti-Glucose-6-phosphate isomerase antibody. Glycogen phosphorylase was detected using the Abcam (ab88078). Monoclonal Anti-α-Tubulin antibody (Sigma–Aldrich) probed with the Polyclonal Rabbit Anti-mouse Immunoglobulins/HRP secondary antibody (Dako Cytomation) were used for the determination of the loading controls.
2.4. Glucose tolerance tests
Glucose tolerance was determined via an Oral Glucose Tolerance Test (OGTT) using 2 g/kg body weight on 6 h fasted mice, as previously described [24], [26]. Glucose tolerance pre- and post-exercise was carried out via an Intraperitoneal Glucose Tolerance Test (IPGTT), on 6-hour fasted mice, as previously described [27].
2.5. Basal turnover, hyperinsulinaemic/euglycaemic clamps and glucose uptake into peripheral tissues
Basal turnover, hyperinsulinaemic/euglycaemic clamps and peripheral glucose uptake into individual tissues under basal and insulin-stimulated conditions were performed in control and KO mice as previously described [28]. Two sets of hyperinsulinaemic clamps were performed with insulin infused at 15 mU/kg/min and 25 mU/kg/min as indicated.
2.6. Plasma and tissue levels
Lactate levels were measured via GM7 Micro – Stat Analyser.
2.7. Muscle and liver glycogen levels
Muscle and liver glycogen levels were assessed by Glycogen Assay kit (ab65620). The assay can detect glycogen 0.0004–2 mg/ml.
2.8. Glucose 6 phosphate (G6P) levels
G6P levels in muscle tissues (white quadriceps) were quantified via the colourimetric G6P (ab83426, Abcam). The G6P kit can detect in the range of 1–30 nmoles with detection sensitivity around 10 μM of G6P.
2.9. Exercise experiments
Prior to performing the exercise experiments, all the experimental and control mice were acclimatised to running on the treadmill (Columbus Instruments International, Exer 3/6) at days 3 and 2 as previously described [29], [30]. To measure exercise and endurance capacity the experimenter was blinded to the mouse genotype. The exercise protocol utilised was modified from [30]. Briefly, for exercise capacity testing, mice conducted a warm-up at a 10% grade at 10 m/min for 10 min, followed by 0.5 m/min increase every 30 s until mice could not be prompted to continue running. One week later the mice underwent the endurance/low intensity protocol, where they started with 10-min 10 m/min warm-up followed by running at 50–60% of their maximal capacity at 0% gradient, until fatigue. The third high-intensity protocol, was conducted one week after the endurance protocol, after a 6-hour fast and included 10-min, 10 m/min warm-up followed by exercise at the same relative intensity (75–85% of the workload maximum as determined from the incremental maximal exercise intensity test) for 30 min or fatigue.
2.10. Statistics
All data are presented as mean ± SEM. ANOVA was used to assess the effect of fasting and insulin during the hyperinsulinaemic clamps glycogen and tissue glucose uptake respectively. Comparisons between single parameters measured were made using the two-tailed, unpaired, Student's t-test (Excel 2013 for Windows). Significance was determined as p < 0.05 according to the analyses.
3. Results
3.1. Muscle specific knockdown of muscle glycogen synthase
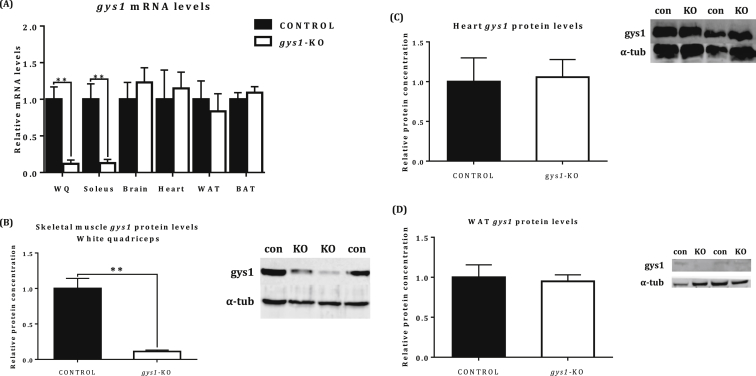
The CRE/Lox system was used to produce time and tissue specific deletion of the gys1 gene in skeletal muscle. To confirm the level of gys1 deletion, we measured mRNA expression levels in white quadriceps and soleus (skeletal muscle), brain, cardiac muscle (heart) and white adipose tissue (WAT) in the gys1-KO (gys1(lox/lox) – cre(+/−)) in comparison with control mice (gys1(lox/lox) – cre(−/−)). Greater than 80% deletion at the level of mRNA was shown in skeletal muscle of the gys1-KO mice compared with the control, both in white [white quadriceps] (Figure 2A, p<0.05 vs. control) and red [soleus] (p<0.05 vs. control) muscle. There was no significant difference in brain, heart, WAT and BAT (Figure 2A) between the two groups, confirming the tissue specificity of the Myosin Light Chain (MLC) 1F skeletal muscle-specific promoter. Assessment of glycogen synthase protein levels via immunoblotting showed 85% reduction in gys1-KO mice compared with the control in skeletal muscle (white quadriceps) (Figure 2B, p<0.001 vs. control). In contrast, no difference was found in heart or WAT (Figure 2C,D).
Figure 2.
Muscle specific knockdown of muscle glycogen synthase. (A) Gys1 mRNA expression levels normalized to 18s rRNA housekeeping gene and shown as change from control in white quadriceps (WQ), soleus, brain, heart, white adipose tissue (WAT) and brown adipose tissue (BAT). (B) Gys1 protein expression levels in white quadriceps (skeletal muscle), (C) heart, and (D) WAT from control and gys1- KO mice at 20–22 weeks of age. Data shown are mean ± SEM (**p < 0.01 vs. control mice, n = 4–8 per group).
3.2. Physiological assessment of gys1-KO mice
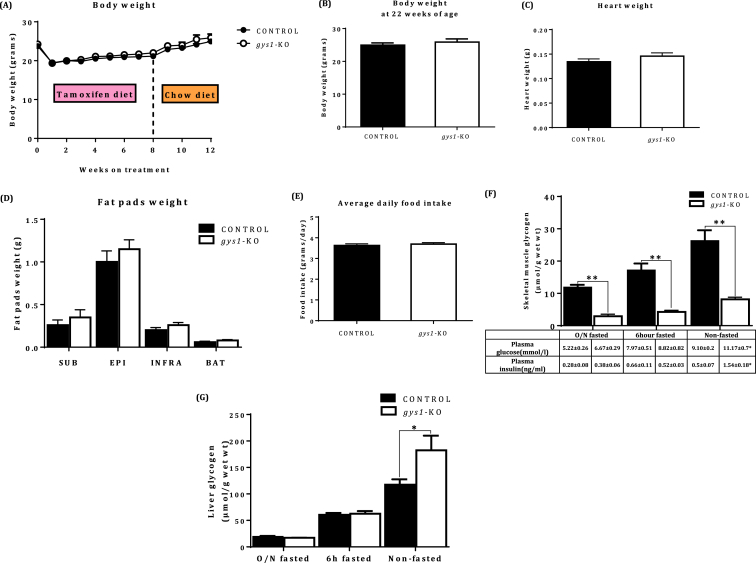
There were no significant differences between the groups in body weight (Figure 3A, B), heart weight (Figure 3C), fat pad weight (Figure 3D), or food intake (Figure 3E). There was no difference in 6-hour or overnight (O/N) fasted plasma glucose or insulin levels (Table in Figure 3F). However, there were statistically significant differences in non-fasted plasma glucose and insulin levels with the gys1-KO group presenting postprandial hyperglycaemia and hyperinsulinaemia, indicative of insulin resistance (Table in Figure 3F, p < 0.05 vs. control). Skeletal muscle glycogen levels were significantly decreased in the gys1-KO mice in the 6-hour and O/N fasted state respectively compared with the control group (Figure 3F, p < 0.05 vs. control). In contrast, liver glycogen levels were 68% higher in gys1-KO mice under non fasting conditions but no significant differences were seen following a 6 h or overnight fast respectively (Figure 3G, p < 0.05 vs. control). For both muscle and liver glycogen levels there was a significant effect of fasting in both control mice (muscle: p < 0.01; liver: p < 0.0001) and gys1-KO mice (muscle: p < 0.005; liver: p < 0.0005).
Figure 3.
Physiological assessment of gys1 KO mice. (A) Body weights from the 10th week of age following tamoxifen induction for 8 weeks followed by a recovery period of 4 weeks on tamoxifen-free chow diet from both control and gys1-KO mice. Data shown are mean ± SEM (n = 10–30 per group). (B) Body weights from 22 week old control and gys1-KO mice. Data shown are mean ± SEM (n = 10–30 per group), (C) Heart weights, (D) Fat pad weights from 22 week old control and gys1-KO mice. Data shown are mean ± SEM (n = 8–10 per group), (E) Average daily food intake from the 10th week of age following tamoxifen induction for 8 weeks from control and gys1-KO mice. Data shown are mean ± SEM (n = 8–10 per group), (F) Skeletal muscle glycogen concentration with plasma glucose and insulin levels under overnight (O/N) fasting, 6 h fasting conditions and non-fasting conditions from control and gys1-KO mice at 22 weeks of age. Data shown are mean ± SEM (**p < 0.01 vs. control, n = 6–7 per group for glycogen, n = 9–11 for glucose & insulin values), (G) Liver glycogen levels under overnight (O/N) fasting, 6 h fasting conditions and non-fasting conditions from control and gys1-KO mice at 22 weeks of age. Data shown are mean ± SEM (*p < 0.05 vs. control mice, n = 4–7 per group).
3.3. Glucose tolerance in gys1 – KO mice
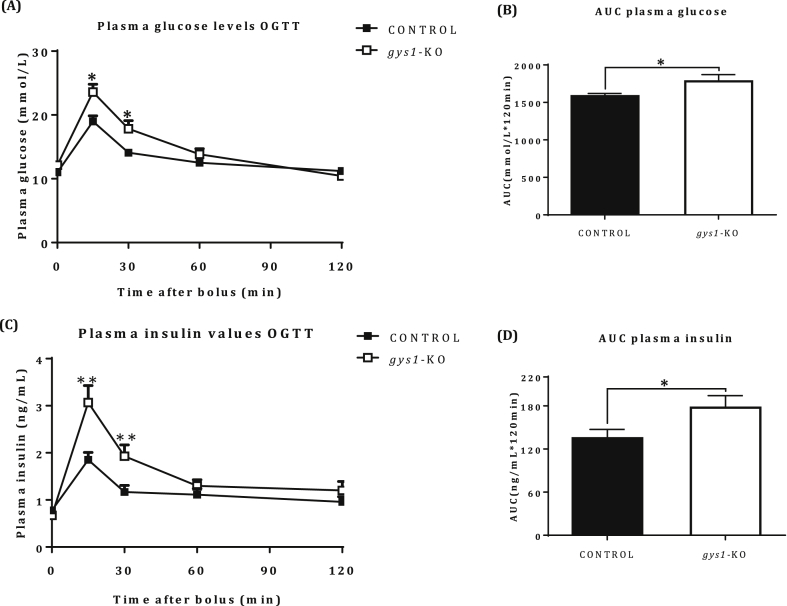
Glucose tolerance was impaired in gys1-KO compared with control mice (Figure 4A, p < 0.05 vs. control, Figure 4B, p < 0.05 vs. control). In addition, the insulin excursion curve (Figure 4C, p < 0.05 vs. control) and AUC for insulin (Figure 4D, p < 0.05 vs. control) showed hyperinsulinaemia in gys1-KO mice, indicative of insulin resistance.
Figure 4.
Glucose tolerance in gys1-KO mice. (A) Plasma glucose excursion curve, (B) Area Under Curve (AUC) from glucose excursion curve, (C) Plasma insulin excursion curve and (D) AUC from insulin excursion curve, following an OGTT under anaesthetized state (2 g/kg of glucose, 6 h fast) over 120 min from control and gys 1-KO mice at 18–22 weeks of age. Data shown are mean ± SEM (*p < 0.05, p < 0.01 vs. control mice, n = 12–13 per group).
3.4. Whole-body insulin sensitivity and glucose metabolism of the gys1-KO mice
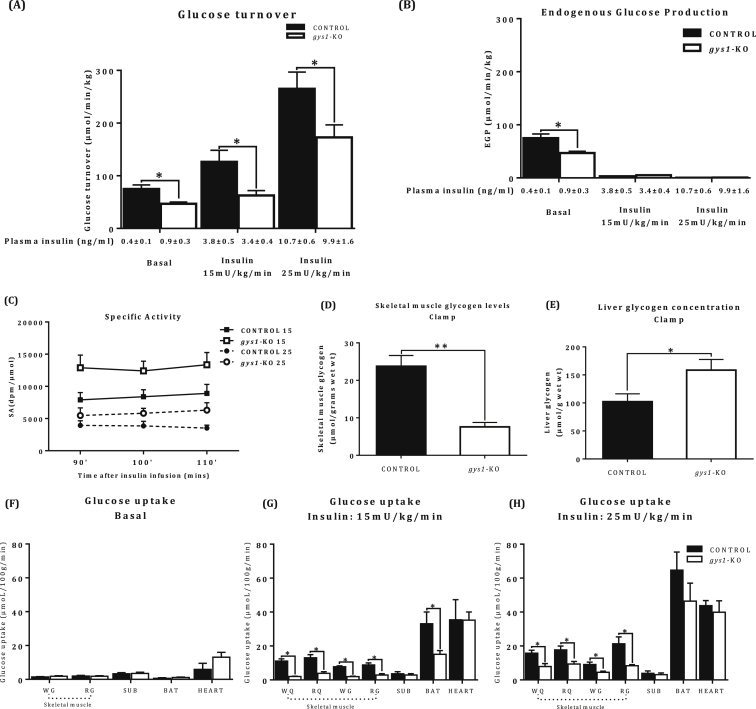
Whole body glucose metabolism, measured using glucose tracer kinetics was carried out both under basal and hyperinsulinaemic conditions. Under basal conditions (Table 1A), the rate of glucose turnover was reduced in gys1-KO mice compared with the control group (Figure 5A, p < 0.05 vs. control). Experiments under hyperinsulinaemic conditions were conducted with a physiological level of insulin (Plasma insulin concentration = 3 ng/ml) (see Figure 4C for physiological postprandial insulin levels) and at a higher insulin stimulation of 10 ng/ml. Under both levels of insulin stimulation, the gys1-KO group showed significantly lower rates of glucose turnover (RD) in comparison with the control group (Figure 5A, p < 0.05 vs. control), at matched plasma glucose and insulin concentrations (Table 1B and C). When the percentage change from basal was calculated, there was no difference at either the lower insulin infusion rate (169.1 ± 15% and 133.5 ± 12% control and muscle GS KO mice respectively) or the higher insulin infusion rate (354.4 ± 22% and 369.1 ± 30% control and muscle GS KO mice respectively). Endogenous glucose production (EGP) was totally suppressed and not different between the two groups (Figure 5B). Steady state specific activity was achieved in all groups undergoing clamp studies (Figure 5C). Skeletal muscle glycogen was 60% reduced in the gys1-KO mice under insulin-stimulated conditions, while liver glycogen was increased (Figure 5D,E, p < 0.05 vs. control). In summary, the glucose turnover data indicate that muscle-specific gys1 deletion results in reduced whole body and muscle specific glucose metabolism under insulin-stimulated conditions, indicative of insulin resistance.
Table 1.
Plasma glucose and plasma insulin levels under 6 h fasting and (A) basal conditions (B) insulin-stimulated conditions (Insulin: 15 mU/kg/min 4 ng/ml of insulin, (C) insulin-stimulated conditions (Insulin: 25 mU/kg/min 10 ng/ml of insulin, in control and gys1-KO mice. Data shown are pooled mean ± SEM (*p < 0.05 vs. control n = 6–8 per group). –SSA: Steady State Average (Average of results from blood samples at 90′, 100′, 110′).
| Condition |
6-hour Fasting |
Basal turnover |
||
|---|---|---|---|---|
| Group | CONTROL | gys1-KO | CONTROL | gys1-KO |
| Plasma glucose (mmol/l) | 8.21 ± 0.60 | 9.26 ± 0.85 | 5.82 ± 0.32 | 5.63 ± 0.34 |
| Plasma insulin (ng/ml) | 0.48 ± 0.06 | 0.64 ± 0.13 | 0.41 ± 0.07 | 0.88 ± 0.26* |
| Hyperinsulinaemic-euglycaemic clamp – Insulin:15mU/kg/min | ||||
|---|---|---|---|---|
| Condition |
6-hour Fasting |
Clamp (SSA) |
||
| Group | CONTROL | gys1-KO | CONTROL | gys1-KO |
| Plasma glucose (mmol/l) | 9.90 ± 0.38 | 12.01 ± 0.95 | 9.24 ± 0.47 | 8.90 ± 0.49 |
| Plasma insulin (ng/ml) | 0.89 ± 0.19 | 1.16 ± 0.20 | 3.82 ± 0.47 | 3.42 ± 0.42 |
| Hyperinsulinaemic-euglycaemic clamp – Insulin:25mU/kg/min | ||||
|---|---|---|---|---|
| Condition |
6-hour Fasting |
Clamp (SSA) |
||
| Group | CONTROL | gys1-KO | CONTROL | gys1-KO |
| Plasma glucose (mmol/l) | 10.91 ± 1.21 | 11.26 ± 0.73 | 11.25 ± 0.84 | 10.57 ± 0.14 |
| Plasma insulin (ng/ml) | 1.01 ± 0.28 | 1.59 ± 0.18 | 10.71 ± 0.60 | 9.90 ± 1.57 |
Figure 5.
Whole-body insulin sensitivity and glucose metabolism of the gys1-KO mice Tissue specific glucose uptake in gys1-KO mice. (A) Rate of glucose turnover and (B) endogenous glucose production under basal and insulin stimulated conditions under two different levels of insulin stimulation (Insulin: 15 and 25 mU/kg/min). (C) Tracer specific activity during the euglycaemic hyperinsulinaemic clamp studies. (D) Skeletal muscle glycogen levels and (E) Liver glycogen levels under insulin-stimulated conditions (F) basal glucose uptake in skeletal muscle [Red gastrocnemius (RG), white gastrocnemius (WG)], WAT from subcutaneous fat (SUB), brown adipose tissue (BAT) and heart (HEART). (G) insulin stimulated glucose uptake (Insulin: 15 mU/kg/min) in skeletal muscle [RG, WG, red quadriceps(RQ), and white quadriceps(WQ)], SUB, BAT and HEART. (H) insulin stimulated glucose uptake (Insulin: 25 mU/kg/min) in skeletal muscle (RG, WG,RQ,WQ), SUB, BAT and HEART after a 6 h fasting period from control and gys1-KO mice at 22 weeks of age. Data shown are mean ± SEM (*p < 0.05 **p < 0.01 vs. control mice, n = 6–8 per group).
3.5. Tissue specific glucose uptake in gys1-KO mice
Glucose uptake into skeletal muscle was significantly elevated in both groups following insulin stimulation in comparison with their respective basal states (Figure 5F,G,H, p < 0.05 vs. basal). Under basal conditions, tissue glucose uptake was not different between the groups (Figure 5F), despite a significant increase in plasma insulin levels in the gys1-KO mice compared with control mice (0.9 ± 0.3 vs. 0.4 ± 0.1 ng/ml, p < 0.05), indicative of insulin resistance. Under insulin-stimulated conditions, the gys1-KO group showed decreased skeletal muscle and brown adipose tissue glucose uptake in comparison with the control (Figure 5G,H, p < 0.05 vs. control) under both levels of insulin stimulation. There were no differences in insulin-stimulated cardiac and WAT glucose uptake between the groups. Furthermore, there was a significant effect of insulin to elicit an increase in tissue glucose uptake (over basal) in all tissues (except WAT) in both control mice (p < 0.005 for tissues tested) and gys1-KO mice (p < 0.005 for all tissues tested). Together, these results show that in the absence of gys1 in skeletal muscle, there is reduced muscle and brown adipose tissue glucose uptake.
3.6. Muscle metabolite and enzyme level quantification
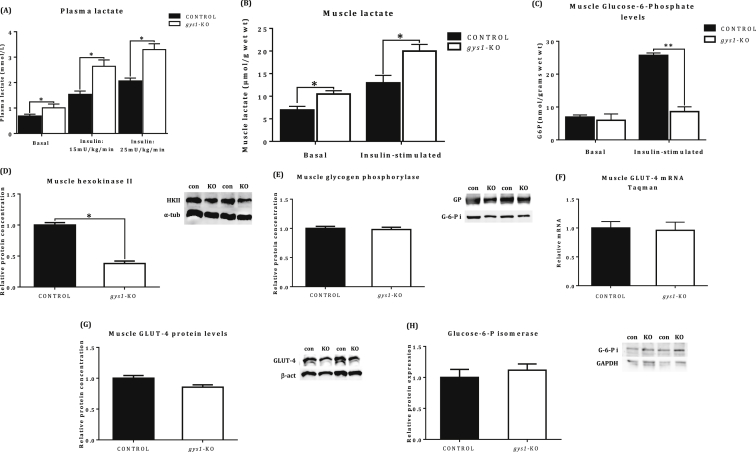
gys1-KO mice presented increased plasma lactate levels under basal and insulin stimulated conditions compared with the control group (Figure 6A, p < 0.05 vs. control). In addition, muscle lactate was also increased in gys1-KO mice under insulin-stimulated conditions (Figure 6B, p < 0.05 vs. control). Additional mechanistic studies showed decreased skeletal muscle glucose-6-phophate levels under both insulin-stimulated conditions (Figure 6D, p < 0.05) and reduced hexokinase II protein levels (Figure 6E, p < 0.05). In contrast, no difference was seen between the groups in glycogen phosphorylase protein concentration (Figure 6F), GLUT-4 mRNA (Figure 6G) and glucose-6-phosphate isomerase protein levels (Figure 6I). There was a trend for lower GLUT-4 protein levels but it did not reach statistical significance (Figure 6H, p = 0.075).
Figure 6.
Muscle metabolite and enzyme level quantification. (A) Plasma lactate levels, (B) Skeletal muscle lactate, (C) Muscle Glucose-6-Phosphate levels under basal and insulin-stimulated conditions. (D) Skeletal muscle hexokinase II, (E) Skeletal muscle glycogen phosphorylase, (F) Skeletal muscle GLUT-4 mRNA, (G) Skeletal muscle GLUT-4 protein, (H) Glucose-6-Phosphate Isomerase levels under insulin-stimulated conditions after a 6 h fasting period from control and gys1-KO mice at 22 weeks of age. Data shown are mean ± SEM (*p < 0.05 **p < 0.01 vs. control mice, n = 4–7 per group).
3.7. Exercise and endurance capacity
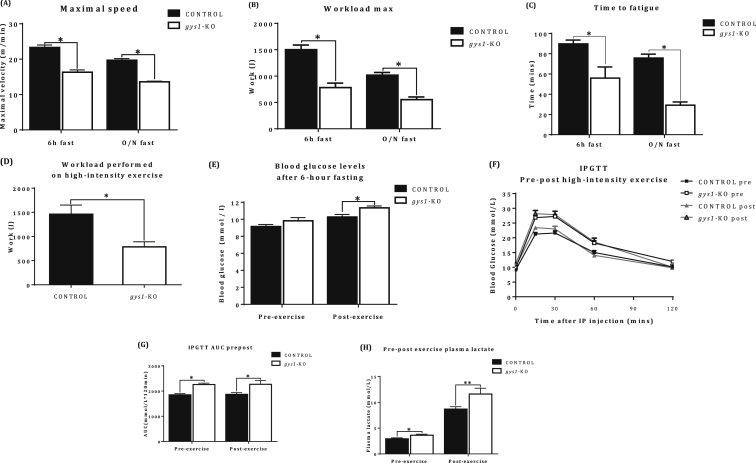
gys1-KO mice showed reduced maximal velocity and maximal workload in comparison to the control group both after 6 h and an overnight fast (Figure 7A,B, p < 0.05 vs. control). Furthermore, gys1-KO mice presented with impaired endurance capacity, reaching “fatigue” much earlier in comparison with control mice (Figure 7C, p < 0.05 vs. control). Finally, following high-intensity exercise gys1-KO mice showed significantly reduced workload in comparison with control mice (Figure 7D, p < 0.05 vs. control). In summary, gys1-KO mice were characterised by impaired exercise and endurance capacity, both at high and low exercise intensity.
Figure 7.
Exercise and endurance capacity. (A) Maximal velocity. (B) Maximal workload as assessed via an incremental exercise protocol. (C) Endurance capacity as estimated from time to exhaustion in a low intensity exercise protocol. (D) Workload performed in a high-intensity exercise protocol (75–80% VO2 max). (E) Pre- and post-exercise blood glucose levels. (F) Glucose Excursion curve. (G) Area Under Curve (AUC) from glucose excursion curve following a pre- and post-exercise conscious IPGTT (2 g/kg of glucose, 6 h fast) over 120 min. (H) Pre-post exercise plasma lactate after a 6 h fasting period from control and gys1-KO mice at 22 weeks of age. Data shown are mean ± SEM (*p < 0.05 **p < 0.01 vs. control mice, n = 14–16 per group).
3.8. Pre-post exercise assessment of glucose tolerance
Glucose tolerance tests were performed prior to the initiation of the exercise protocol and immediately following the last bout of exercise, which was the high intensity protocol. This was done to assess the insulin-independent effects of exercise on glucose tolerance. gys1-KO mice showed post-exercise hyperglycaemia in comparison with the control group, while there was no difference in glycaemia in the pre-exercise, 6-hour fasted blood glucose levels (Figure 7E, p < 0.05 vs. control). Furthermore, gys1-KO displayed pre and post-exercise glucose intolerance in comparison with the control. One bout of high-intensity exercise did not have a significant effect on glucose tolerance in either group (Figure 7F,G, p < 0.05 vs. control). Plasma lactate levels were also higher in gys1-KO mice pre and post exercise (Figure 7H).
4. Discussion
In an effort to elucidate the role of muscle glycogen synthase in glucose metabolism and insulin resistance, we generated a conditional muscle-specific deletion of gys1. To our knowledge, there is no existing animal model of skeletal muscle-specific gys1 deletion in adulthood, which more accurately reflects an acquired defect in muscle glucose metabolism. Our inducible and muscle specific gys1-KO mice displayed normal body weight, heart weight, fat pad weight and food intake, which is in contrast with the global KO mice that were 5–10% smaller than control [20]. In addition, our muscle-specific gys1-KO mice showed postprandial hyperglycaemia and hyperinsulinaemia, indicating impaired glucose metabolism under non-fasting conditions. In contrast, the surviving global gys1-KO mice showed no change in glucose and insulin levels in the fed or fasted state and indeed better glucose tolerance, which contradicts our results. We confirmed decreased insulin sensitivity and muscle glucose uptake in our gys1-KO mice with hyperinsulinaemic/euglycaemic clamps. Furthermore, reduced muscle hexokinase II protein levels in our novel muscle-specific gys1-KO model are in agreement with previous findings in insulin resistance and T2D [31], [32], [33]. Our results support a number of human studies associating gys1 defects with impairments in insulin sensitivity and whole body glucose metabolism [34], [35].
In terms of muscle glycogen and exercise, strong association has been suggested between muscle glycogen depletion, impaired muscle performance and fatigue development during exercise [15], [36], [37], [38], [39]. The principal reason leading to fatigue during exercise is not clear and many different mechanisms have been proposed. Some of these, supported by the “glycogen shunt” hypothesis of Shulman and Rothman [16] suggest that glycogen depletion may lead to fatigue, indicating that glycogen is the principal energy source for muscle contraction and that muscle glycogen is required for blood glucose to enter glycolysis. Specifically, exercise leads to reduced muscle glycogen content in both control and patients with type 2 diabetes, with a concomitant increase in glycogen synthase activity [40]. When glycogen stores are depleted with endurance exercise, fat mobilisation becomes an important source of energy [41], [42]. Furthermore, exercise results in increased muscle GLUT4 protein levels [43], [44], PDK-4 and UCP-3 mRNA [45] and IL-6 mRNA levels associated with nuclear phosphorylated p38 MAPK [46]. These studies in humans suggest that glycogen can associate with and regulate the expression of proteins important in the generation of the glycogen granule and glycogen synthesis [47]. In our study, we found that muscle hexokinase II (HKII) protein levels were reduced in the gys1-KO mice. Whether this is directly or indirectly due to a reduction in muscle glycogen levels is not clear. Previous investigations have shown that exercise can increase muscle HKII mRNA more so than GLUT4 [48], [49]. Furthermore, overexpression of HKII resulted in exercise and insulin-stimulated increases in muscle glucose transport [50], whereas heterozygous deletion of this enzyme impaired exercise capacity [51]. Whether the reduction in HKII expression (in the presence of normal GLUT4 expression) contributes to the impaired glucose uptake and exercise capacity in our animal model, as has previously been suggested [52], remains to be determined. We also found increased plasma lactate levels pre- and post-exercise, which could also contribute to impaired exercise capacity.
In contrast to the current findings in humans, Pederson et al. demonstrated that in mice, muscle glycogen is not essential for exercise [22], suggesting that rodents may not need muscle glycogen and may depend on liver glycogen stores for exercise. This can be supported by the argument that muscle glycogen, expressed as a relative percentage of body mass, is significantly lower (10-fold) in mice in comparison to humans [53], [54], [55]. In contrast, liver glycogen levels are relatively similar/comparable between rodents and humans [54]. Based on these findings, Pederson et al. hypothesized that the relative roles of muscle and liver glycogen may be different between the two species. However, in our research, we attribute these findings to the complex phenotype of the model and not to the difference between humans and rodents. We show that muscle glycogen is a critical factor for high-intensity, intermittent and lower intensity exercise, as has previously been shown [37], [38], [39], [56]. This is also supported by the findings in patients with Glycogen Storage Disease 0b, whose symptoms include muscle weakness, pain, cramps and poor exercise performance [17], [18]. Furthermore, several genetically modified mouse lines with altered muscle and liver metabolism have been studied for exercise and endurance capacity. Over-accumulation of skeletal muscle glycogen because of overexpression of a hyperactive form of gys1 did not result in increased muscle glucose uptake following stimulation [57], nor did it improve exercise performance [58]. In support of this finding, transgenic RGL (regulatory-targeting subunit of the muscle-specific glycogen-associated protein phosphatase PP1G) mice, that were characterised by increased muscle glycogen levels, also had normal exercise tolerance [59]. However, muscle-specific overexpression of PPAR1γ coactivator-1a (PGC-1a) that increased muscle glycogen levels did increase exercise performance [60]. In contrast, muscle GLUT4 KO (normal muscle glycogen content), liver glycogen synthase (gys2 – normal muscle glycogen content) KO and muscle RGL KO (reduced muscle glycogen content) mice displayed reduced exercise capacity and performance [59], [61], [62], [63]. In our model, inducible muscle-specific gys1-KO mice were characterised by impaired exercise and endurance capacity, highlighting the importance of skeletal muscle glycogen in exercise and endurance capacity. However, we did not see an effect of exercise on glucose tolerance in either the control or gys-1-KO mice. Previous studies have shown that, following exercise, there is an increase in both whole body glucose appearance and disappearance when challenged with an oral glucose load [64]. The majority of the glucose appearance was from the oral load, possibly due to exercise-induced changes in intestinal absorption and/or reduced liver glucose uptake to ensure appropriate glucose supply to the brain. The reason that we did not see an increase in the glucose excursion curves immediately following exercise is not clear and warrants further investigation. Interestingly, while the absolute muscle glycogen levels were lower in the gys-1 KO mice, the response to fasting was similar between the two groups of mice. Indeed, the same was seen in the percent increase in glucose turnover with increasing insulin during the clamp studies. This suggests that despite an inducible deletion of muscle gys1, the mice do have the ability to respond to physiological stimuli.
Given the importance of glycogen synthesis to glucose disposal it is surprising that there is only one transgenic, one allosterically mutated “knock-in” and one KO animal model targeting muscle glycogen synthase, without a clear picture of the consequences on glucose metabolism and exercise capacity. Rabbit gys1 over expression [19] in mouse skeletal muscle resulted in augmentation of glycogen synthase activity (10-fold) and glycogen content (up to 5 fold), reinforcing that gys1 has a crucial role in muscle glycogen synthesis. The increase in muscle glycogen levels occurred due to increased gys1 and not secondary to the capacity of glucose transport as previously suggested [65]. However, physiological assessment of these transgenic mice was not conducted. The allosterically mutated mice showed normal glucose metabolism despite a decrease in glycogen synthesis and muscle glycogen content [23]. On the other hand, global gys1 deletion [20], [21] led to 90% perinatal mortality due to impaired cardiac function, oedema, pooling of blood, and haemorrhagic liver. Unexpectedly, the surviving mice showed normal cardiac morphology and function and, remarkably, improved glucose tolerance. As gys1 was globally deleted its absence in tissues other than skeletal muscle probably led to a complex and most likely compensated for phenotype, resulting in improved glucose tolerance despite reduced muscle glucose uptake in these surviving animals. One can envision that inhibition of muscle glycogen synthesis due to gys1 deletion can lead to glucose being metabolised via other pathways including glycolysis (non-oxidative glucose metabolism) leading to lactate production or oxidised via the Krebs cycle leading to the production of energy in the form of ATP. An increase of glucose flux through the hexosamine biosynthesis pathway is also possible [66] and all of these scenarios could contribute to a complex phenotype. Furthermore, the global gys1-KO was generated on a mixed background strain (C57BL/6J and 129/SvJ), which may also provide a valid explanation for the variations in phenotype. It is well known that different strains of mice have genetic differences that could significantly impact physiological outcomes [67]. Our current mice were generated using C57BL/6J embryonic stem cells and consequently maintained on the C57BL/6J background, which is the most widely used reference strain in metabolism and T2D [68].
One of the more intriguing finding in metabolism is that the body replenishes and deposits liver glycogen only when the glycogen replete muscle releases excess carbons in the form of lactate. The liver preferentially deposits glycogen from glucose newly synthesised via gluconeogenesis [69]. In this study, deletion of gys1 specifically in skeletal muscle was achieved in adult mice using an inducible muscle specific CRE mouse mated to a mouse harbouring a floxed gys1. This model has confirmed the primary role of muscle glycogen storage in the clearance of a glucose load and has shown why muscle glycogen is given precedence over liver glycogen. Muscle is unable to take up sufficient glucose from the circulation when rapid muscle contraction is required. In the battle for survival, the need for rapid locomotion meant that locally stored glucose was needed. This is also brain protective since any increased capacity of the muscle to take up blood glucose would put a further strain on the liver to supply sufficient glucose to the brain.
In summary, the present study suggests that gys1 plays a major role in muscle glycogen synthesis and non-oxidative glucose metabolism as a muscle-specific gys1 deletion in the adult mouse can contribute to peripheral insulin resistance and glucose intolerance. In addition, gys1 is crucial for muscle function during contraction, exercise and endurance capacity as muscle glycogen depletion is a leading factor in impaired muscle performance and fatigue development during exercise.
Author contribution
Conceptualization: JP, JDZ and SA; Methodology: CEX, KB, ZR, BWT, BL, JF, WP, SA, JP and JDZ; Validation: CEX, ZR, SPM, and SA Format Analysis: CEX; Investigation: CEX, SA, SPM, ZR, AH, AB and BL, Writing –Original Draft: CEX and SA; Writing – Review & Editing: JP, BL and SPM; Visualization: CEX and SA; Supervision: SA, JP, and JDZ Project Administration: SA, and JP; Funding Acquisition: SA, JDZ and JP.
Acknowledgements
The authors would like to thank Alexandra Funkat, Blaise Henrich, Margaret Shaw, Lisa Billington, Rebecca Sgambellone and Yaelle Faulsen for their excellent technical assistance and contribution to the molecular and physiological characterisation of the model. This work was supported by Diabetes Australia Research Program and National Health and Medical Research Council of Australia Project Grant.
Conflict of interest
We declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
References
- 1.DeFronzo R., Jacot E., Jequier E., Maeder E., Wahren J., Felber J. The effect of insulin on the disposal of intravenous glucose: results from indirect calorimetry and hepatic and femoral venous catheterization. Diabetes. 1981;30:1000–1007. doi: 10.2337/diab.30.12.1000. [DOI] [PubMed] [Google Scholar]
- 2.DeFronzo R.A., Ferrannini E., Sato Y., Felig P., Wahren J. Synergistic interaction between exercise and insulin on peripheral glucose uptake. Journal of Clinical Investigation. 1981;68:1468. doi: 10.1172/JCI110399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jue T., Rothman D.L., Shulman G.I., Tavitian B.A., DeFronzo R.A., Shulman R.G. Direct observation of glycogen synthesis in human muscle with 13C NMR. Proceedings of the National Academy of Sciences. 1989;86:4489–4491. doi: 10.1073/pnas.86.12.4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abdul-Ghani M.A., DeFronzo R.A. Pathogenesis of insulin resistance in skeletal muscle. Journal of Biomedicine and Biotechnology. 2010;2010:19. doi: 10.1155/2010/476279. Article ID 476279, http://dx.doi.org/10.1155/2010/476279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DeFronzo R.A., Tripathy D. Skeletal muscle insulin resistance is the primary defect in type 2 diabetes. Diabetes Care. 2009;32:S157–S163. doi: 10.2337/dc09-S302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shulman G.I., Rothman D.L., Jue T., Stein P., DeFronzo R.A., Shulman R.G. Quantitation of muscle glycogen synthesis in normal subjects and subjects with non-insulin-dependent diabetes by 13C nuclear magnetic resonance spectroscopy. New England Journal of Medicine. 1990;322:223–228. doi: 10.1056/NEJM199001253220403. [DOI] [PubMed] [Google Scholar]
- 7.Utriainen T., Takala T., Luotolahti M., Rönnemaa T., Laine H., Ruotsalainen U. Insulin resistance characterizes glucose uptake in skeletal muscle but not in the heart in NIDDM. Diabetologia. 1998;41:555–559. doi: 10.1007/s001250050946. [DOI] [PubMed] [Google Scholar]
- 8.Pendergrass M., Bertoldo A., Bonadonna R., Nucci G., Mandarino L., Cobelli C. Muscle glucose transport and phosphorylation in type 2 diabetic, obese nondiabetic, and genetically predisposed individuals. American Journal of Physiology: Endocrinology and Metabolism. 2007;292:E92–E100. doi: 10.1152/ajpendo.00617.2005. [DOI] [PubMed] [Google Scholar]
- 9.Bogardus C., Thuillez P., Ravussin E., Vasquez B., Narimiga M., Azhar S. Effect of muscle glycogen depletion on in vivo insulin action in man. Journal of Clinical Investigation. 1983;72:1605. doi: 10.1172/JCI111119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bogardus C., Lillioja S., Howard B., Reaven G., Mott D. Relationships between insulin secretion, insulin action, and fasting plasma glucose concentration in nondiabetic and noninsulin-dependent diabetic subjects. Journal of Clinical Investigation. 1984;74:1238. doi: 10.1172/JCI111533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bogardus C., Lillioja S., Mott D., Reaven G., Kashiwagi A., Foley J. Relationship between obesity and maximal insulin-stimulated glucose uptake in vivo and in vitro in Pima Indians. Journal of Clinical Investigation. 1984;73:800. doi: 10.1172/JCI111274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bogardus C., Lillioja S., Stone K., Mott D. Correlation between muscle glycogen synthase activity and in vivo insulin action in man. Journal of Clinical Investigation. 1984;73:1185. doi: 10.1172/JCI111304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yki-Järvinen H., Mott D., Young A., Stone K., Bogardus C. Regulation of glycogen synthase and phosphorylase activities by glucose and insulin in human skeletal muscle. Journal of Clinical Investigation. 1987;80:95. doi: 10.1172/JCI113069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roach P., Depaoli-Roach A., Hurley T., Tagliabracci V. Glycogen and its metabolism: some new developments and old themes. The Biochemical Journal. 2012;441:763–787. doi: 10.1042/BJ20111416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hermansen L., Hultman E., Saltin B. Muscle glycogen during prolonged severe exercise. Acta Physiologica Scandinavica. 1967;71:129–139. doi: 10.1111/j.1748-1716.1967.tb03719.x. [DOI] [PubMed] [Google Scholar]
- 16.Shulman R., Rothman D. The “glycogen shunt” in exercising muscle: a role for glycogen in muscle energetics and fatigue. Proceedings of the National Academy of Sciences. 2001;98:457–461. doi: 10.1073/pnas.98.2.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kollberg G., Tulinius M., Gilljam T., Ostman-Smith I., Forsander G., Jotorp P. Cardiomyopathy and exercise intolerance in muscle glycogen storage disease 0. New England Journal of Medicine. 2007;357:1507–1514. doi: 10.1056/NEJMoa066691. [DOI] [PubMed] [Google Scholar]
- 18.Sukigara S., Liang W.-C., Komaki H., Fukuda T., Miyamoto T., Saito T. Muscle glycogen storage disease 0 presenting recurrent syncope with weakness and myalgia. Neuromuscular Disorders. 2012;22:162–165. doi: 10.1016/j.nmd.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 19.Manchester J., Skurat A.V., Roach P., Hauschka S.D., Lawrence J.C. Increased glycogen accumulation in transgenic mice overexpressing glycogen synthase in skeletal muscle. Proceedings of the National Academy of Sciences. 1996;93:10707–10711. doi: 10.1073/pnas.93.20.10707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pederson B.A., Chen H., Schroeder J.M., Shou W., DePaoli-Roach A.A., Roach P.J. Abnormal cardiac development in the absence of heart glycogen. Molecular and Cellular Biology. 2004;24:7179–7187. doi: 10.1128/MCB.24.16.7179-7187.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pederson B.A., Schroeder J.M., Parker G.E., Smith M.W., DePaoli-Roach A.A., Roach P.J. Glucose metabolism in mice lacking muscle glycogen synthase. Diabetes. 2005;54:3466–3473. doi: 10.2337/diabetes.54.12.3466. [DOI] [PubMed] [Google Scholar]
- 22.Pederson B.A., Cope C.R., Schroeder J.M., Smith M.W., Irimia J.M., Thurberg B.L. Exercise capacity of mice genetically lacking muscle glycogen synthase in mice, muscle glycogen is not essential for exercise. Journal of Biological Chemistry. 2005;280:17260–17265. doi: 10.1074/jbc.M410448200. [DOI] [PubMed] [Google Scholar]
- 23.Bouskila M., Hunter R.W., Ibrahim A.F., Delattre L., Peggie M., van Diepen J.A. Allosteric regulation of glycogen synthase controls glycogen synthesis in muscle. Cell Metabolism. 2010;12:456–466. doi: 10.1016/j.cmet.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 24.Andrikopoulos S., Blair A.R., Deluca N., Fam B.C., Proietto J. Evaluating the glucose tolerance test in mice. American Journal of Physiology: Endocrinology and Metabolism. 2008;295:E1323–E1332. doi: 10.1152/ajpendo.90617.2008. [DOI] [PubMed] [Google Scholar]
- 25.Kebede M., Favaloro J., Gunton J.E., Laybutt D.R., Shaw M., Wong N. Fructose-1, 6-bisphosphatase overexpression in pancreatic β-cells results in reduced insulin secretion a new mechanism for fat-induced impairment of β-cell function. Diabetes. 2008;57:1887–1895. doi: 10.2337/db07-1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fam B.C., Rose L.J., Sgambellone R., Ruan Z., Proietto J., Andrikopoulos S. Normal muscle glucose uptake in mice deficient in muscle GLUT4. Journal of Endocrinology. 2012;214:313–327. doi: 10.1530/JOE-12-0032. [DOI] [PubMed] [Google Scholar]
- 27.Lamont B.J., Visinoni S., Fam B.C., Kebede M., Weinrich B., Papapostolou S. Expression of human fructose-1, 6-bisphosphatase in the liver of transgenic mice results in increased glycerol gluconeogenesis. Endocrinology. 2006;147:2764–2772. doi: 10.1210/en.2005-1498. [DOI] [PubMed] [Google Scholar]
- 28.Lamont B.J., Andrikopoulos S., Funkat A., Favaloro J., Ye J.M., Kraegen E.W. Peripheral insulin resistance develops in transgenic rats overexpressing phosphoenolpyruvate carboxykinase in the kidney. Diabetologia. 2003;46:1338–1347. doi: 10.1007/s00125-003-1180-y. [DOI] [PubMed] [Google Scholar]
- 29.Huijsman E., van de Par C., Economou C., van der Poel C., Lynch G.S., Schoiswohl G. Adipose triacylglycerol lipase deletion alters whole body energy metabolism and impairs exercise performance in mice. American Journal of Physiology: Endocrinology and Metabolism. 2009;297:E505–E513. doi: 10.1152/ajpendo.00190.2009. [DOI] [PubMed] [Google Scholar]
- 30.Steinberg G.R., O'Neill H.M., Dzamko N.L., Galic S., Naim T., Koopman R. Whole body deletion of AMP-activated protein kinase β2 reduces muscle AMPK activity and exercise capacity. Journal of Biological Chemistry. 2010;285:37198–37209. doi: 10.1074/jbc.M110.102434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Braithwaite S.S., Palazuk B., Colca J.R., Edwards C.W., Hofmann C. Reduced expression of hexokinase II in insulin-resistant diabetes. Diabetes. 1995;44:43–48. doi: 10.2337/diab.44.1.43. [DOI] [PubMed] [Google Scholar]
- 32.Kruszynska Y.T., Mulford M.I., Baloga J., Yu J.G., Olefsky J.M. Regulation of skeletal muscle hexokinase II by insulin in nondiabetic and NIDDM subjects. Diabetes. 1998;47:1107–1113. doi: 10.2337/diabetes.47.7.1107. [DOI] [PubMed] [Google Scholar]
- 33.Pendergrass M., Koval J., Vogt C., Yki-Jarvinen H., Iozzo P., Pipek R. Insulin-induced hexokinase II expression is reduced in obesity and NIDDM. Diabetes. 1998;47:387–394. doi: 10.2337/diabetes.47.3.387. [DOI] [PubMed] [Google Scholar]
- 34.Thoburn A., Gumbiner B., Bulacan F., Wallace P., Henry R. Intracellular glucose oxidation and glycogen synthesis activity are reduced in non-insulin-dependent (type II) diabetes independent of impaired glucose uptake. Journal of Clinical Investigation. 1990;85:522–529. doi: 10.1172/JCI114468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thorburn A., Gumbiner B., Bulacan F., Brechtel G., Henry R. Multiple defects in muscle glycogen synthase activity contribute to reduced glycogen synthesis in non-insulin dependent diabetes mellitus. Journal of Clinical Investigation. 1991;87:489. doi: 10.1172/JCI115022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bergström J., Hermansen L., Hultman E., Saltin B. Diet, muscle glycogen and physical performance. Acta Physiologica Scandinavica. 1967;71:140–150. doi: 10.1111/j.1748-1716.1967.tb03720.x. [DOI] [PubMed] [Google Scholar]
- 37.Holloszy J.O., Kohrt W.M., Hansen P.A. The regulation of carbohydrate and fat metabolism during and after exercise. Frontiers in Bioscience. 1998;3:D1011–D1027. doi: 10.2741/a342. [DOI] [PubMed] [Google Scholar]
- 38.Ivy J.L. Role of carbohydrate in physical activity. Clinics in Sports Medicine. 1999;18:469–484. doi: 10.1016/s0278-5919(05)70162-9. [DOI] [PubMed] [Google Scholar]
- 39.Karlsson J., Saltin B. Diet, muscle glycogen, and endurance performance. Journal of Applied Physiology. 1971;31:203–206. doi: 10.1152/jappl.1971.31.2.203. [DOI] [PubMed] [Google Scholar]
- 40.Pedersen A.J., Hingst J.R., Friedrichsen M., Kristensen J.M., Hojlund K., Wojtaszewski J.F. Dysregulation of muscle glycogen synthase in recovery from exercise in type 2 diabetes. Diabetologia. 2015;58:1569–1578. doi: 10.1007/s00125-015-3582-z. [DOI] [PubMed] [Google Scholar]
- 41.Coyle E.F. Substrate utilization during exercise in active people. American Journal of Clinical Nutrition. 1995;61:968S–979S. doi: 10.1093/ajcn/61.4.968S. [DOI] [PubMed] [Google Scholar]
- 42.Jeukendrup A.E., Saris W.H., Wagenmakers A.J. Fat metabolism during exercise: a review. Part I: fatty acid mobilization and muscle metabolism. International Journal of Sports Medicine. 1998;19:231–244. doi: 10.1055/s-2007-971911. [DOI] [PubMed] [Google Scholar]
- 43.Kraniou G.N., Cameron-Smith D., Hargreaves M. Effect of short-term training on GLUT-4 mRNA and protein expression in human skeletal muscle. Experimental Physiology. 2004;89:559–563. doi: 10.1113/expphysiol.2004.027409. [DOI] [PubMed] [Google Scholar]
- 44.Kraniou G.N., Cameron-Smith D., Hargreaves M. Acute exercise and GLUT4 expression in human skeletal muscle: influence of exercise intensity. Journal of Applied Physiology. 2006;1985(101):934–937. doi: 10.1152/japplphysiol.01489.2005. [DOI] [PubMed] [Google Scholar]
- 45.Cluberton L.J., McGee S.L., Murphy R.M., Hargreaves M. Effect of carbohydrate ingestion on exercise-induced alterations in metabolic gene expression. Journal of Applied Physiology. 2005;1985(99):1359–1363. doi: 10.1152/japplphysiol.00197.2005. [DOI] [PubMed] [Google Scholar]
- 46.Chan M.H., McGee S.L., Watt M.J., Hargreaves M., Febbraio M.A. Altering dietary nutrient intake that reduces glycogen content leads to phosphorylation of nuclear p38 MAP kinase in human skeletal muscle: association with IL-6 gene transcription during contraction. FASEB Journal. 2004;18:1785–1787. doi: 10.1096/fj.03-1039fje. [DOI] [PubMed] [Google Scholar]
- 47.Philp A., Hargreaves M., Baar K. More than a store: regulatory roles for glycogen in skeletal muscle adaptation to exercise. American Journal of Physiology: Endocrinology and Metabolism. 2012;302:E1343–E1351. doi: 10.1152/ajpendo.00004.2012. [DOI] [PubMed] [Google Scholar]
- 48.O'Doherty R.M., Bracy D.P., Granner D.K., Wasserman D.H. Transcription of the rat skeletal muscle hexokinase II gene is increased by acute exercise. Journal of Applied Physiology. 1996;1985(81):789–793. doi: 10.1152/jappl.1996.81.2.789. [DOI] [PubMed] [Google Scholar]
- 49.O'Doherty R.M., Bracy D.P., Osawa H., Wasserman D.H., Granner D.K. Rat skeletal muscle hexokinase II mRNA and activity are increased by a single bout of acute exercise. American Journal of Physiology. 1994;266:E171–E178. doi: 10.1152/ajpendo.1994.266.2.E171. [DOI] [PubMed] [Google Scholar]
- 50.Fueger P.T., Bracy D.P., Malabanan C.M., Pencek R.R., Granner D.K., Wasserman D.H. Hexokinase II overexpression improves exercise-stimulated but not insulin-stimulated muscle glucose uptake in high-fat-fed C57BL/6J mice. Diabetes. 2004;53:306–314. doi: 10.2337/diabetes.53.2.306. [DOI] [PubMed] [Google Scholar]
- 51.Fueger P.T., Heikkinen S., Bracy D.P., Malabanan C.M., Pencek R.R., Laakso M. Hexokinase II partial knockout impairs exercise-stimulated glucose uptake in oxidative muscles of mice. American Journal of Physiology: Endocrinology and Metabolism. 2003;285:E958–E963. doi: 10.1152/ajpendo.00190.2003. [DOI] [PubMed] [Google Scholar]
- 52.Wasserman D.H. Four grams of glucose. American Journal of Physiology: Endocrinology and Metabolism. 2009;296:E11–E21. doi: 10.1152/ajpendo.90563.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kasuga M., Ogawa W., Ohara T. Tissue glycogen content and glucose intolerance. Journal of Clinical Investigation. 2003;111:1282. doi: 10.1172/JCI18526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Orho M., Bosshard N.U., Buist N., Gitzelmann R., Aynsley-Green A., Blümel P. Mutations in the liver glycogen synthase gene in children with hypoglycemia due to glycogen storage disease type 0. Journal of Clinical Investigation. 1998;102:507. doi: 10.1172/JCI2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hribal M.L., Oriente F., Accili D. Mouse models of insulin resistance. American Journal of Physiology: Endocrinology and Metabolism. 2002;282:E977–E981. doi: 10.1152/ajpendo.00561.2001. [DOI] [PubMed] [Google Scholar]
- 56.Balsom P.D., Gaitanos G., Söderlund K., Ekblom B. High-intensity exercise and muscle glycogen availability in humans. Acta Physiologica Scandinavica. 1999;165:337–346. doi: 10.1046/j.1365-201x.1999.00517.x. [DOI] [PubMed] [Google Scholar]
- 57.Fogt D.L., Pan S., Lee S., Ding Z., Scrimgeour A., Lawrence J.C., Jr. Effect of glycogen synthase overexpression on insulin-stimulated muscle glucose uptake and storage. American Journal of Physiology: Endocrinology and Metabolism. 2004;286:E363–E369. doi: 10.1152/ajpendo.00115.2003. [DOI] [PubMed] [Google Scholar]
- 58.Pederson B.A., Cope C.R., Irimia J.M., Schroeder J.M., Thurberg B.L., Depaoli-Roach A.A. Mice with elevated muscle glycogen stores do not have improved exercise performance. Biochemical and Biophysical Research Communications. 2005;331:491–496. doi: 10.1016/j.bbrc.2005.03.206. [DOI] [PubMed] [Google Scholar]
- 59.Aschenbach W.G., Suzuki Y., Breeden K., Prats C., Hirshman M.F., Dufresne S.D. The muscle-specific protein phosphatase PP1G/RGL (GM) is essential for activation of glycogen synthase by exercise. Journal of Biological Chemistry. 2001;276:39959–39967. doi: 10.1074/jbc.M105518200. [DOI] [PubMed] [Google Scholar]
- 60.Calvo J.A., Daniels T.G., Wang X., Paul A., Lin J., Spiegelman B.M. Muscle-specific expression of PPARγ coactivator-1α improves exercise performance and increases peak oxygen uptake. Journal of Applied Physiology. 2008;104:1304–1312. doi: 10.1152/japplphysiol.01231.2007. [DOI] [PubMed] [Google Scholar]
- 61.Irimia J.M., Meyer C.M., Peper C.L., Zhai L., Bock C.B., Previs S.F. Impaired glucose tolerance and predisposition to the fasted state in liver glycogen synthase knock-out mice. Journal of Biological Chemistry. 2010;285:12851–12861. doi: 10.1074/jbc.M110.106534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Howlett K.F., Andrikopoulos S., Proietto J., Hargreaves M. Exercise-induced muscle glucose uptake in mice with graded, muscle-specific GLUT-4 deletion. Physiological Reports. 2013;1:e00065. doi: 10.1002/phy2.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Muoio D.M., MacLean P.S., Lang D.B., Li S., Houmard J.A., Way J.M. Fatty acid homeostasis and induction of lipid regulatory genes in skeletal muscles of peroxisome proliferator-activated receptor (PPAR) α knock-out mice evidence for compensatory regulation by PPARδ. Journal of Biological Chemistry. 2002;277:26089–26097. doi: 10.1074/jbc.M203997200. [DOI] [PubMed] [Google Scholar]
- 64.Rose A.J., Howlett K., King D.S., Hargreaves M. Effect of prior exercise on glucose metabolism in trained men. y: Endocrinology and Metabolism. 2001;281:E766–E771. doi: 10.1152/ajpendo.2001.281.4.E766. [DOI] [PubMed] [Google Scholar]
- 65.Suzuki Y., Lanner C., Kim J.-H., Vilardo P.G., Zhang H., Yang J. Insulin control of glycogen metabolism in knockout mice lacking the muscle-specific protein phosphatase PP1G/RGL. Molecular and Cellular Biology. 2001;21:2683–2694. doi: 10.1128/MCB.21.8.2683-2694.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Randle P.J., Kerbey A.L., Espinal J. Mechanisms decreasing glucose oxidation in diabetes and starvation: role of lipid fuels and hormones. Diabetes/Metabolism Reviews. 1988;4:623–638. doi: 10.1002/dmr.5610040702. [DOI] [PubMed] [Google Scholar]
- 67.Andrikopoulos S., Massa C.M., Aston-Mourney K., Funkat A., Fam B.C., Hull R.L. Differential effect of inbred mouse strain (C57BL/6, DBA/2, 129T2) on insulin secretory function in response to a high fat diet. Journal of Endocrinology. 2005;187:45–53. doi: 10.1677/joe.1.06333. [DOI] [PubMed] [Google Scholar]
- 68.Clee S.M., Attie A.D. The genetic landscape of type 2 diabetes in mice. Endocrine Reviews. 2007;28:48–83. doi: 10.1210/er.2006-0035. [DOI] [PubMed] [Google Scholar]
- 69.Newgard C.B., Moore S., Foster D., McGarry J. Efficient hepatic glycogen synthesis in refeeding rats requires continued carbon flow through the gluconeogenic pathway. Journal of Biological Chemistry. 1984;259:6958–6963. [PubMed] [Google Scholar]