Figure 1.
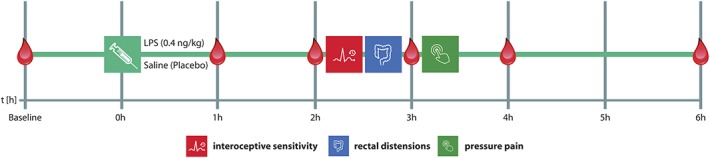
Study design: In this randomized, double-blind, placebo-controlled crossover study, healthy men (N = 20) and women using hormonal contraceptives (N = 20) received an intravenous injection of either lipopolysaccharide (LPS; 0.4 ng/kg body weight) or the same volume of saline (placebo) on 2 separate study days in a randomized order. Assessments of interoceptive sensitivity (accuracy of heartbeat perception), visceral thresholds (pressure-controlled rectal distensions), and musculoskeletal pain sensitivity (pressure pain thresholds) were conducted 2 to 3.5 hours after injection of LPS or placebo. Plasma samples for cytokine and cortisol analyses were collected at baseline as well as 1, 2, 3, 4, and 6 hours after injection, followed by assessment of state anxiety.
