High- and low-frequency transcutaneous electrical nerve stimulation is effective for reducing experimental heat pain in young individuals but not in older individuals.
Keywords: Transcutaneous electrical nerve stimulation (TENS), Pain, Hypoalgesia, Aging, Elderly, Rehabilitation, Physical therapy, Physiotherapy, Pain threshold, Conditioned pain modulation, Segmental analgesia
Abstract
Despite its widespread clinical use, the efficacy of transcutaneous electrical nerve stimulation (TENS) remains poorly documented in elderly individuals. In this randomized, double-blind crossover study, we compared the efficacy of high-frequency (HF), low-frequency (LF), and placebo (P) TENS in a group of 15 elderly adults (mean age: 67 ± 5 years). The effect of HF-, LF-, and P-TENS was also evaluated in a group of 15 young individuals (26 ± 5 years; same study design) to validate the effectiveness of the TENS protocols that were used in the elderly group. Each participant came to the laboratory on 3 separate occasions to receive, in random order, HF-, LF-, and P-TENS. Pain intensity and pain perception thresholds were assessed before, during, and after TENS, using an experimental heat pain paradigm. For the young group, there was a significant decrease in pain intensity during and after HF- and LF-TENS when compared with baseline, with both HF- and LF-TENS being superior to P-TENS. In the older group, HF- and LF-TENS did not reduce pain when compared with baseline and no difference was observed between the 2 active TENS sessions and P-TENS. High-frequency, LF-, and P-TENS all increased pain thresholds in young individuals, whereas in older individuals, only LF-TENS increased pain thresholds. Taken together, these results suggest that TENS is effective in young, but not in older, individuals. Future studies should be conducted to confirm these results in pain populations and to identify strategies that could enhance the effect of TENS in the elderly.
1. Introduction
Chronic pain is a prevalent health care condition, affecting approximately 100 million adults in the United States.15 The prevalence of chronic pain significantly increases with age, with more than 50% of elderly people reporting persistent pain.23,49,57 According to the American Geriatrics Society, seniors suffering from persistent pain should receive both pharmacological and nonpharmacological treatment options.1 However, the efficacy of many nonpharmacological approaches used today in older individuals has yet to be confirmed.48
Transcutaneous electrical nerve stimulation (TENS) is a nonpharmacological modality that is commonly used in rehabilitation to reduce pain.52 The most common TENS parameters used are high-frequency, low-intensity stimulations (>10 Hz, comfortable intensity; high-frequency TENS [HF-TENS]) and low-frequency, high-intensity stimulations (<10 Hz, strong intensity; low-frequency TENS [LF-TENS]).13,25 High-frequency transcutaneous electrical nerve stimulations allow depolarization of Aβ fibers, producing segmental analgesia through gate-control mechanisms.19,41 On the other hand, the strong stimulations induced by LF-TENS depolarize Aδ and C fibers and decrease pain through activation of descending pain modulating mechanisms originating from the brain stem.20,26,35,60 Both HF- and LF-TENS produce their hypoalgesic effect through the release of endogenous opioids, with δ-opioid receptors mediating the hypoalgesia of HF-TENS and μ-opioid receptor mediating the hypoalgesia of LF-TENS.28,30,51,54
Past studies have shown that TENS can help reduce pain,6,25 analgesic consumption, and medication-related side effects.7,18 These advantages are of particular interest for clinicians working with the elderly, a portion of the population who are often heavily medicated and prone to pharmacological side effects.3,50 Unfortunately, studies looking into the clinical efficacy of TENS are mainly performed on young adults or on age-heterogeneous populations, and the clinical efficacy of TENS in the elderly population remains poorly documented. To our knowledge, very few studies have specifically evaluated the hypoalgesic effect of TENS in elderly participants (see, for instance, Refs. 9 22 44). Although interesting, these studies have important limitations (absence of placebo condition, incomplete description of the study's population or of the TENS treatments), hence precluding any final conclusion that can be made regarding the efficacy of TENS in elderly people. The aim of this study was to fill this knowledge gap and determine whether TENS is an effective treatment option for older individuals. More specifically, the objective was to compare the efficacy of HF-, LF-, and placebo (P) TENS in a group of elderly individuals. The effect of HF-, LF-, and P-TENS was also evaluated in a group of young participants to validate the effectiveness of the TENS protocols that were used in this study.
2. Methods
2.1. Participants
Fifteen young adults aged between 21 and 39 years (mean age: 26 ± 5 years; 6 men) and 15 older adults aged between 58 and 74 years (mean age: 67 ± 5 years; 6 men) were included in the study. Participants were excluded if they were pregnant and/or had a pacemaker (TENS contraindications), used opioids in the past 6 months,29,53 or had an existing neurological or pain condition affecting the lumbar region. Every participant was asked to refrain from consuming caffeine38 and short-term analgesics 6 hours before testing and tobacco products 2 hours before testing. The experiment took place at the Research Center on Aging of the Health and Social Services Center, University Institute of Geriatrics of Sherbrooke (Sherbrooke, Quebec, Canada). Subjects were recruited through local advertisements and were all French-speaking community-dwelling individuals. The study was approved by the local institutional ethics committee, and each participant provided informed written consent before participating in the study.
2.2. Experimental design
A double-blind, placebo-controlled crossover design was used. Volunteers received 3 interventions, during 3 separate sessions (1-week interval), in random order: (1) HF-TENS, (2) LF-TENS, and (3) P-TENS. Randomization was performed using a random numbers table, controlling for presentation order. Controlling for presentation order ensured that an equal number of participants were randomized to each possible permutation level. Each time, the tonic heat pain test was performed on 4 occasions: (1) at baseline (T0), (2) during TENS (after 15 minutes of TENS; T1), (3) immediately after TENS (T2), and (4) 30 minutes after TENS application (T3). Heat pain thresholds (HPTs) were also measured at baseline, during TENS (after 15 minutes of TENS), and immediately after TENS to evaluate the effect of TENS on pain sensitivity.
2.3. Tonic heat pain model
Participants were seated comfortably in a massage chair (Fig. 1). A pretesting session was first conducted to familiarize participants with the computerized visual analog scale (CoVAS; Medoc, Advanced Medical Systems, Minneapolis, MN) and to determine the temperature that would be used during the 2-minute tonic heat pain test. This pretest was performed with a 10-cm2 Peltier-type thermode (Medoc, Advanced Medical Systems) applied to the thoracic region. Participants were advised that the thermode temperature would gradually rise from 32°C to 51°C (rising rate = 0.3°C/s). During the first pretest, subjects verbally reported their pain perception threshold and pain tolerance threshold. On the second pretest, subjects were given the CoVAS and advised that they would have to start moving the cursor towards the right (towards the “100” mark) when they would start to feel pain (pain perception threshold) and that the cursor would need to be at the extreme right (at the “100” mark) when pain was intolerable (pain tolerance threshold). This procedure was repeated until the subject's pain reports were consistent between trials. The temperature used during the following experimental heat pain test was determined by selecting the temperature for which the subject had rated the pain intensity at 50 of 100 (moderate pain) with the CoVAS (see Ref. 30 for a similar approach). The decision to use a test stimulus that would produce moderate levels of pain was based on the observations of Benedetti et al.5 who reported that TENS is effective for mild or moderate pain, but not for severe pain.
Figure 1.
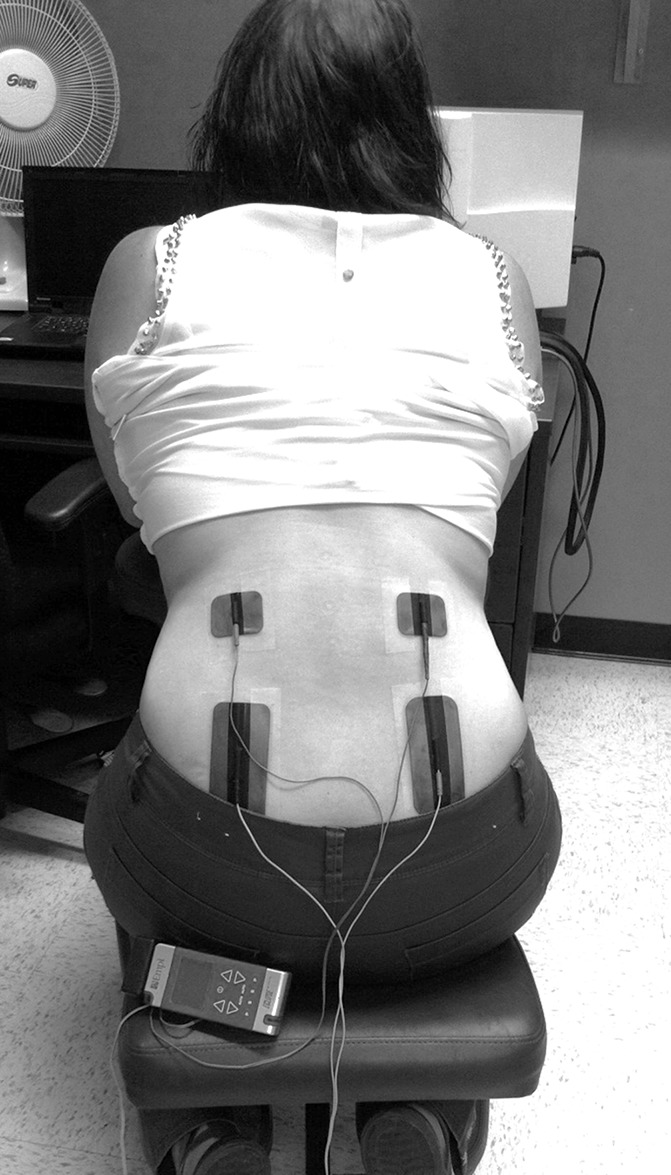
Transcutaneous electrical nerve stimulation application.
After the pretest, participants were given a 10-minute rest period before the experimental test began. The tonic pain test was performed with the application of the thermode at a constant temperature applied on the lumbar region for 2 minutes. Subjects were told that the thermode temperature could rise, remain stable, or decrease and that they would need to evaluate their pain with the CoVAS throughout the 2 minutes of the test. In fact, after a constant rise (1°C/s) from baseline (32°C) to the individually predetermined temperature, the thermode's temperature remained constant throughout the times of the test.
2.4. Heat pain threshold
Heat pain thresholds were evaluated in the lumbar region with the Peltier-type thermode. The threshold was determined using the method of limits.42,61 Participants were advised that the temperature of the thermode would gradually increase and that they would need to report their first pain sensation by clicking on the left button of a computer mouse (baseline = 32°C; rising rate = 1°C/s). A total of 3 HPT values were taken at each time measure. The 3 values of the same time measure were then averaged to obtain a single HPT value.
2.5. TENS protocol
For each visit, TENS were delivered using 2 pairs of rubber silicone electrodes connected to a digital Eclipse Plus apparatus (Empi, St. Paul, Minnesota). Electrodes were placed on the lower thoracic and lumbar region (Fig. 1). For HF-TENS, the frequency was set at 100 Hz and the pulse duration at 60 μs, and the intensity was adjusted to produce strong and comfortable (innocuous) tingling sensations.35,52,58 For LF-TENS, the frequency was set at 3 Hz and the pulse duration at 400 μs, and the intensity was adjusted to produce strong and painful sensations.35,52,58 For P-TENS, the frequency was set at 100 Hz and the pulse duration at 60 μs. However, the TENS apparatus was turned “OFF” using a hidden device, which disabled the electrodes without changing the display on the equipment (electric stimulation applied to built-in resistors). The participants were led to believe that there was an electric current (indication of stimulation on the TENS device), but in reality, electric current was dissipated as heat in the resistors (no electrical stimulation given to the participants). For all TENS conditions, the stimulation was applied for 25 minutes and the intensity was raised for HF- and LF-TENS, if needed, at minutes 10 and 20 of stimulation, based on the participant's sensation, to account for nerve habituation.33,47
2.6. Data analysis
To facilitate comparisons, pain intensity ratings obtained during the 2-minute tonic heat pain test were averaged and the mean was used in subsequent analyses. The study was designed to detect a difference of 20 points on the CoVAS (clinically important difference21). To detect this difference in each age group, with 80% power and a 5% significance level, we determined that 15 young adults and 15 older adults had to be enrolled in the study (estimated SD of 26, based on preliminary results). Given the design of the study, power calculations were made based on within-group analyses. Within-group analyses allowed us to determine both the effect of the independent variables TIME (T0, T1, T2, and T3) and CONDITION (HF-TENS, LF-TENS, and P-TENS). Sample size was calculated using nQuery Advisor (version 4.0., Statistical Solutions, Cork, Ireland).
Because of the small number of subjects and because visual inspection of the histograms did not allow us to assume that the data were normally distributed, nonparametric tests were used. For each TENS treatment (HF-, LF-, and P-TENS), Friedman tests were used to compare the pain scores and the pain thresholds before, during, and after TENS application (TIME variable). This allowed us to determine whether each TENS treatment influenced pain perception. Friedman tests were also used to compare the pain scores and the pain thresholds across the 3 TENS treatments for the same time measure (CONDITION variable). This allowed us to directly compare the efficacy of HF-TENS, LF-TENS, and P-TENS. Differences were considered to be significant if P < 0.05 was obtained. Bonferroni corrections were applied to all post hoc multiple analyses to prevent for type I errors. All tests were performed using SPSS (version 17.0 for Windows, Chicago, IL).
3. Results
3.1. Participant characteristics and stimulation parameters
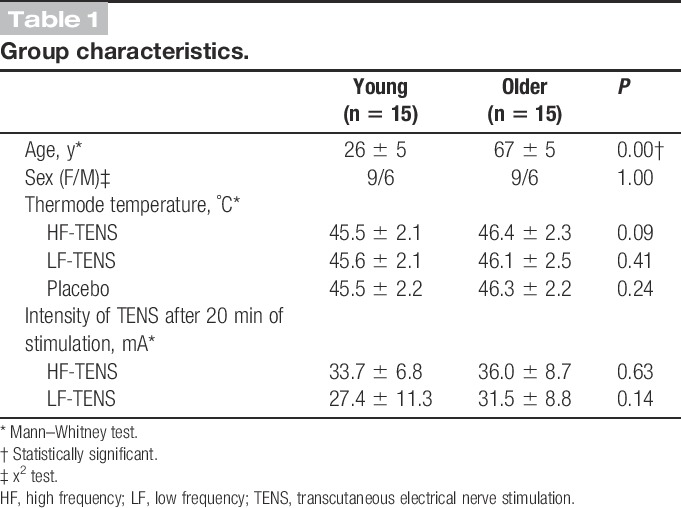
The characteristics of the participants and stimulation parameters are presented in Table 1. Each group was composed of 9 women and 6 men. Of the 30 participants, 6 (3 in the young group and 3 in the older group) identified the presence of a placebo treatment (success rate for blinding of 80%).
Table 1.
Group characteristics.
3.2. Baseline pain measures
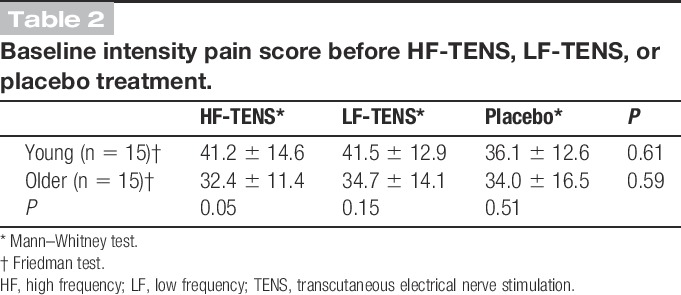
The mean pain intensity ratings obtained at baseline (T0) showed that every participant experienced pain before TENS application (all pain intensity scores >7). The mean thermode pain scores obtained before TENS application are presented in Table 2. As can be seen from the table, baseline pain scores were comparable for the 3 TENS conditions and between the 2 age groups. There was a slight difference between the 2 age groups for the HF-TENS condition. The difference did not however reach statistical (P = 0.05) or clinical significance.21
Table 2.
Baseline intensity pain score before HF-TENS, LF-TENS, or placebo treatment.
3.3. Pain intensity
The average pain intensity scores obtained before, during, and after the different TENS conditions in young individuals are presented in Figure 2A. Pain intensity decreased with both HF- and LF-TENS. Friedman tests and post hoc Wilcoxon signed-rank tests confirmed that there was a significant reduction in pain during (T1) and after TENS application (T2 and T3) in young individuals when compared with baseline for both HF- and LF-TENS (all P < 0.01). The reduction in pain was both statistically and clinically significant (pain reduction >20 points21). No change was observed after P-TENS (P = 0.28). A significant difference was observed at T1 between HF- and P-TENS and between LF- and P-TENS (all P ≤ 0.05). No difference was observed between HF- and LF-TENS at T1 (P = 1.0) and between the 3 TENS conditions at T2 and T3 (all P > 0.29).
Figure 2.
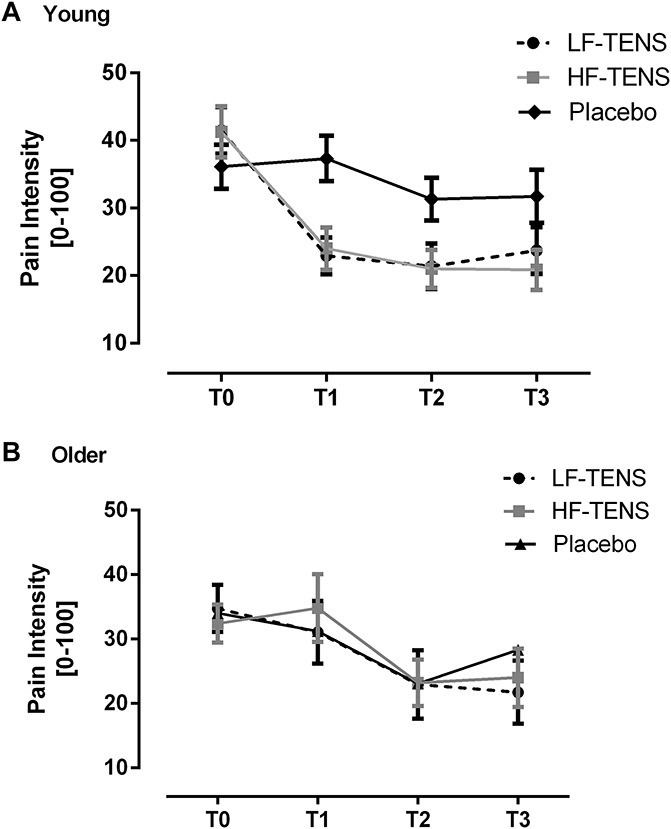
Pain intensity before (T0), during (T1), immediately after (T2), and 30 minutes after transcutaneous electrical nerve stimulation (TENS) application (T3) in young (A) and older (B) participants. When compared with baseline, there was a significant reduction in pain during and after high-frequency and low-frequency TENS in young (all P ≤ 0.01) but not in older individuals (all P ≥ 0.1). For the young group, a significant difference was observed at T1 between high-frequency and placebo TENS and between low-frequency and placebo TENS (all P ≤ 0.05). No significant difference was observed between the 3 TENS conditions in the older group (all P > 0.20).
The average pain intensity scores obtained before, during, and after the different TENS conditions in older individuals are shown in Figure 2B. When compared with baseline, there was no change in pain during and after TENS application (all P ≥ 0.07). No significant difference was observed between the 3 TENS conditions at T1, T2, and T3 (all P ≥ 0.20).
3.4. Heat pain threshold
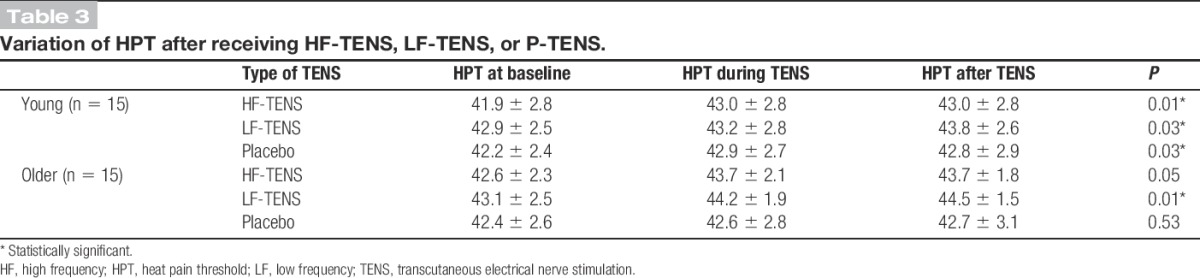
The HPT values obtained before, during, and after TENS are presented in Table 3. In the young group, Friedman tests showed that HF-, LF-, and P-TENS all modified HPT. Post hoc Wilcoxon signed-rank tests revealed that HPT increased during and after HF-TENS when compared with baseline (all P < 0.01). For LF-TENS, HPT increased after (P < 0.05), but not during (P = 0.69) TENS application. For P-TENS, HPT increased during (P < 0.05), but not after (P = 0.19) TENS application. In the older group, Friedman tests showed that LF-TENS, but not HF- and P-TENS, altered HPT. Post hoc Wilcoxon signed-rank tests revealed that HPT increased during and after LF-TENS when compared with baseline (all P < 0.05).
Table 3.
Variation of HPT after receiving HF-TENS, LF-TENS, or P-TENS.
3.5. Conditioning effect and expectations
Because previous studies have observed that the conditioning effects provided by the experience of placebos can influence the results of clinical trials,2,30,31 we performed between-subject analyses to determine whether the hypoalgesic response observed after HF- and LF-TENS applications was influenced by the order of presentation. To do this, delta pain scores, representing pain reductions experienced after HF- and LF-TENS applications (delta pain score = pain at baseline − pain after TENS), were calculated and compared between participants who received P-TENS during their first session and those who received HF- and LF-TENS during their first session. The analyses revealed that the order of presentation did not influence the pattern of results (ie, similar hypoalgesia after HF- and LF-TENS for participants who received P-TENS during their first visit and participants who received HF- or LF-TENS during their first visit; P > 0.53).
In an attempt to evaluate the perceived plausibility of the placebo intervention, we further evaluated the participants' expectations regarding the different TENS protocols (measured by asking the participants to give a percentage of their expected pain relief). The Friedman test revealed that the expectations were similar for HF-, LF-, and P-TENS for both the young and older groups (all P > 0.23), suggesting that the placebo condition was seen as a plausible intervention by the participants.
4. Discussion
In this study, we evaluated the hypoalgesic effect of HF-, LF-, and P-TENS in a group of young and older individuals. The analyses of pain intensity ratings obtained during the experimental heat pain paradigm revealed a strong and significant decrease in pain for HF- and LF-TENS in young individuals. The important hypoalgesic effect noted in young adults contrasts with the absence of hypoalgesic effect noted in older participants. In this latter group, there was no change in pain for both HF- and LF-TENS, indicating that neither one of these TENS paradigms is effective for reducing experimental pain in elderly individuals. For the pain thresholds, we also observed a differential pattern of responses between young and older individuals, with young participants showing increased pain thresholds for HF-, LF-, and P-TENS and older participants showing increased pain thresholds for LF-TENS only, suggesting, once again, that the hypoalgesic effect of TENS is altered in older individuals.
4.1. TENS effect in young individuals
Many of the previous studies looking into the hypoalgesic effect of TENS were conducted with young or age-heterogeneous populations. For example, in their study, Chesterton et al.,12 evaluated the effect of HF- and LF-TENS on mechanical pain thresholds in a group of 240 healthy young subjects (mean age: 30 ± 7 years). The authors reported that HF- and LF-TENS similarly increased pain thresholds, suggesting that these 2 TENS protocols are effective for reducing pain in young adults. These results somewhat contrast with the results of Chen and Johnson,11 who noted a greater effect on mechanical pain thresholds for HF-TENS compared with LF-TENS. In opposition to Chesterton et al.12 (who applied LF-TENS at a strong/to tolerance intensity level), Chen and Johnson11 applied LF-TENS at a low/nonpainful intensity level. We believe that LF-TENS should be applied at strong/painful stimulation intensities.13,36,60
To our knowledge, 6 studies have evaluated the effect of TENS using experimental heat pain paradigms.8,32,46,55,56,59 Although the quality of these studies was generally low, the majority (5 of 6 studies) found positive effects of TENS (see, for instance, Ref. 13 for a commendable review on the effect of TENS on experimental pain). The hypoalgesic effect of TENS has also been studied directly in clinical pain populations, with some studies showing positive34,37 and other studies showing negative16 results. In 2007, Jonhson and Martinson25 performed a large meta-analysis, regrouping 38 studies with various musculoskeletal pain populations, to determine whether the hypoalgesic effect of TENS is superior to that of placebo. The meta-analysis showed that TENS is more effective than placebo, with the authors suggesting that the equivocal results reported in previous studies may have been due to insufficient statistical power.
4.2. TENS effect in older individuals
To our knowledge, very few studies have specifically looked into the hypoalgesic effect of TENS in elderly individuals (see, for instance, Refs. 22 44). In their studies, Grant et al.22 and Ng et al.44 both reported positive effects of TENS on pain in older patients. Yet, it is important to note that the studies of Grant et al.22 and Ng et al.44 did not include a placebo condition. It is therefore impossible to determine whether the effect observed after TENS application by these authors is attributable to an active treatment component.4
The present results, in particular the ones regarding the absence of hypoalgesic effect of LF-TENS in older individuals, are in line with the results of Edwards et al.17 and Lariviere et al.27 who observed reduced efficacy of descending pain inhibition in older individuals compared with young individuals. Indeed, it should be kept in mind that the hypoalgesic effect of LF-TENS depends on the activation of descending pain modulating mechanisms originating from the brain stem.20,26,35,60 The results of this study confirm and extend the results of Edwards et al.17 and Larivière et al.27 by showing that the efficacy of descending and segmental pain mechanisms is affected in older individuals.
4.3. Effect of TENS on heat pain thresholds
Previous reports have shown that HF- and LF-TENS can increase pain threshold (including HPT,8 cold pain threshold,10 and mechanical pain threshold).12,55 In this study, we observed an increased HPT with HF-, LF-, and P-TENS in young participants and an increase in HPT with LF-TENS (but not with HF- and P-TENS) in older participants. Although our observations are somewhat in line with the results of Cheing and Hui-Chan8 (who observed increased HPT in young individuals after HF- and LF-TENS) and with the results of Chesterton et al.12 (who observed increased pain thresholds in young individuals after HF- and LF-TENS), it remains difficult to explain why the results obtained with HPT differ from those obtained with the tonic heat pain test. These discrepancies can probably be explained by the different mechanisms involved. For instance, detection of HPT is believed to rely on the activity of Aδ fibers, while the pain experienced during tonic nociceptive stimuli mostly depends on C-fiber activation.36 The results obtained from a study by Naert et al.,43 who observed that tonic heat pain ratings only moderately correlated with HPT, support such an interpretation. Accordingly, our results tend to suggest that the effect of LF-TENS on the nociceptive activity of the Aδ fibers is more robust than that of HF-TENS, at least in elderly individuals.
4.4. Increased baseline pain scores
Although nonsignificant, analysis of baseline pain scores revealed that elderly participants tended to experience slightly lower pain before TENS application compared with young participants. In a past research report, Benedetti et al. showed that baseline pain scores could affect TENS efficacy. In their study, Benedetti et al. noted a positive effect of TENS for patients with mild or moderate pain, but not for those with severe pain. These results suggest that high pain levels can negatively affect TENS efficacy. In this study, we observed that the hypoalgesic effect of HF-TENS observed in young individuals was absent in older individuals despite the fact that the later reported slightly lower baseline pain scores than the former. Hence, the group difference in baseline pain scores does not jeopardize the conclusions regarding the absent TENS response noted in elderly individuals.
4.5. Potential neurophysiological mechanisms
The hypoalgesic effect of TENS depends on the activation of opioid and nonopioid circuits located at the spinal and supraspinal level.26,30,35,41,54 Some authors have reported significant age-related changes in these spinal14,24 and supraspinal circuits.39,45 For instance, Hoskins and colleagues observed reduced spinal opioid-induced antinociception responses in older rats,14 a finding that could be explained by the age-related changes in the affinity of spinal opioid receptors.24 These findings could help to explain the blunted TENS response observed in elderly individuals. Future research is necessary to better understand the neurophysiological mechanisms underlying the results observed in this study.
4.6. Limitations
One potential limitation that could be addressed to this study is the use of an experimental pain paradigm rather than a clinical pain paradigm. Without refuting the fact that experimental pain paradigms have less external validity than clinical pain paradigms, the former has, on the counterpart, the advantage of increasing internal validity. For example, in this study, using an experimental pain paradigm allowed us to evaluate the hypoalgesic effect of TENS in young and older individuals who experienced comparable pain (ie, nociceptive/thermal pain, moderate intensity level). We believe that recruiting young and older participants with similar pain conditions and profiles would have been a very difficult, if not impossible, task. Having 2 groups of participants with different pain profiles would certainly have reduced our ability to make clear assertions regarding the similarities/differences of young and older individuals. Nevertheless, we must keep in mind that the results of this study cannot be directly generalized to clinical pain conditions. More studies need to be conducted in pain populations before any definitive conclusion can be made regarding the effect of TENS in the elderly.
Another important limitation concerns the relatively low statistical power observed for the older group. Indeed, contrarily to the analyses for the young group (73% < 1 − β < 100%), the analyses made in the older group reached a statistical power situated between 10% and 65%. This situation can be explained by the high variability of pain responses measured in older participants, a situation that has also been reported for other types of measures.40,62 The lower statistical power observed in the older group increases the chances of the occurrence of a type II error. However, one has to remember that the hypoalgesic response observed in older participants was not clinically significant (reduction in pain scores <20 points). Therefore, even if statistically significant, the reduction in pain observed in elderly individuals after HF- and LF-TENS would have been of little clinical importance.
5. Conclusion
In this study, we demonstrated that elderly individuals do not respond to TENS as well as young adults. In particular, we observed that, although effective for reducing pain in young adults, both HF- and LF-TENS did not significantly reduce pain ratings in the elderly. These observations can offer possible explanations for the contradictory results that are sometimes observed in the literature concerning TENS effectiveness. Clearly, more studies should be conducted to confirm the present results in pain populations and to identify strategies that could enhance TENS hypoalgesia in the elderly.
Conflict of interest statement
The authors have no conflicts of interest to declare.
G. Léonard and H. Corriveau are supported by Fonds de recherche Santé (FRQ-S, Québec, Canada). G. Léonard is supported by the Natural Sciences and Engineering Research Council (NSERC, Canada). H. Corriveau is supported by the Canadian Institutes of Health Research (CIHR).
Acknowledgements
The authors thank Ms. Marie-Claude Girard and Mr. Mathieu Hamel for their help with data collection. They also thank all the subjects who participated in this project. Part of this work served as an MSc degree fulfillment by Kayla Bergeron-Vézina.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
References
- [1].American Geriatrics Society. Pharmacological management of persistent pain in older persons. Pain Med 2009;10:1062–83. [DOI] [PubMed] [Google Scholar]
- [2].Andre-Obadia N, Magnin M, Garcia-Larrea L. On the importance of placebo timing in rTMS studies for pain relief. PAIN 2011;152:1233–7. [DOI] [PubMed] [Google Scholar]
- [3].Ballentine NH. Polypharmacy in the elderly: maximizing benefit, minimizing harm. Crit Care Nurs Q 2008;31:40–5. [DOI] [PubMed] [Google Scholar]
- [4].Beaulieu P, Lussier D, Porreca F, Dickenson AH. Pharmacology of pain. Seattle: IASP Press, 2010. [Google Scholar]
- [5].Benedetti F, Amanzio M, Casadio C, Cavallo A, Cianci R, Giobbe R, Mancuso M, Ruffini E, Maggi G. Control of postoperative pain by transcutaneous electrical nerve stimulation after thoracic operations. Ann Thorac Surg 1997;63:773–6. [DOI] [PubMed] [Google Scholar]
- [6].Bjordal JM, Johnson MI, Ljunggreen AE. Transcutaneous electrical nerve stimulation (TENS) can reduce postoperative analgesic consumption. A meta-analysis with assessment of optimal treatment parameters for postoperative pain. Eur J Pain 2003;7:181–8. [DOI] [PubMed] [Google Scholar]
- [7].Chabal C, Fishbain DA, Weaver M, Heine LW. Long-term transcutaneous electrical nerve stimulation (TENS) use: impact on medication utilization and physical therapy costs. Clin J Pain 1998;14:66–73. [DOI] [PubMed] [Google Scholar]
- [8].Cheing GL, Hui-Chan CW. Analgesic effects of transcutaneous electrical nerve stimulation and interferential currents on heat pain in healthy subjects. J Rehabil Med 2003;35:15–19. [DOI] [PubMed] [Google Scholar]
- [9].Cheing GL, Hui-Chan CW, Chan KM. Does four weeks of TENS and/or isometric exercise produce cumulative reduction of osteoarthritic knee pain? Clin Rehabil 2002;16:749–60. [DOI] [PubMed] [Google Scholar]
- [10].Chen CC, Johnson MI. A comparison of transcutaneous electrical nerve stimulation (TENS) at 3 and 80 pulses per second on cold-pressor pain in healthy human participants. Clin Physiol Funct Imaging 2010;30:260–8. [DOI] [PubMed] [Google Scholar]
- [11].Chen CC, Johnson MI. An investigation into the hypoalgesic effects of high- and low-frequency transcutaneous electrical nerve stimulation (TENS) on experimentally-induced blunt pressure pain in healthy human participants. J Pain 2010;11:53–61. [DOI] [PubMed] [Google Scholar]
- [12].Chesterton LS, Barlas P, Foster NE, Lundeberg T, Wright CC, Baxter GD. Sensory stimulation (TENS): effects of parameter manipulation on mechanical pain thresholds in healthy human subjects. PAIN 2002;99:253–62. [DOI] [PubMed] [Google Scholar]
- [13].Claydon LS, Chesterton LS, Barlas P, Sim J. Dose-specific effects of transcutaneous electrical nerve stimulation (TENS) on experimental pain: a systematic review. Clin J Pain 2011;27:635–47. [DOI] [PubMed] [Google Scholar]
- [14].Crisp T, Stafinsky JL, Hoskins DL, Dayal B, Chinrock KM, Uram M. Effects of aging on spinal opioid-induced antinociception. Neurobiol Aging 1994;15:169–74. [DOI] [PubMed] [Google Scholar]
- [15].Deyo RA, Dworkin SF, Amtmann D, Andersson G, Borenstein D, Carragee E, Carrino J, Chou R, Cook K, DeLitto A, Goertz C, Khalsa P, Loeser J, Mackey S, Panagis J, Rainville J, Tosteson T, Turk D, Von Korff M, Weiner DK. Report of the National Institutes of Health Task Force on research standards for chronic low back pain. J Manipulative Physiol Ther 2014;37:449–67. [DOI] [PubMed] [Google Scholar]
- [16].Deyo RA, Walsh NE, Martin DC, Schoenfeld LS, Ramamurthy S. A controlled trial of transcutaneous electrical nerve stimulation (TENS) and exercise for chronic low back pain. N Engl J Med 1990;322:1627–34. [DOI] [PubMed] [Google Scholar]
- [17].Edwards RR, Fillingim RB, Ness TJ. Age-related differences in endogenous pain modulation: a comparison of diffuse noxious inhibitory controls in healthy older and younger adults. PAIN 2003;101:155–65. [DOI] [PubMed] [Google Scholar]
- [18].Elvir-Lazo OL, White PF. The role of multimodal analgesia in pain management after ambulatory surgery. Curr Opin Anaesthesiol 2010;23:697–703. [DOI] [PubMed] [Google Scholar]
- [19].Ersek RA. Transcutaneous electrical neurostimulation: a new therapeutic modality for controlling pain. Clin Orthop Relat Res 1977;128:314–24. [PubMed] [Google Scholar]
- [20].Facchinetti F, Sandrini G, Petraglia F, Alfonsi E, Nappi G, Genazzani AR. Concomitant increase in nociceptive flexion reflex threshold and plasma opioids following transcutaneous nerve stimulation. PAIN 1984;19:295–303. [DOI] [PubMed] [Google Scholar]
- [21].Farrar JT, Portenoy RK, Berlin JA, Kinman JL, Strom BL. Defining the clinically important difference in pain outcome measures. PAIN 2000;88:287–94. [DOI] [PubMed] [Google Scholar]
- [22].Grant DJ, Bishop-Miller J, Winchester DM, Anderson M, Faulkner S. A randomized comparative trial of acupuncture versus transcutaneous electrical nerve stimulation for chronic back pain in the elderly. PAIN 1999;82:9–13. [DOI] [PubMed] [Google Scholar]
- [23].Helme RD, Gibson SJ. The epidemiology of pain in elderly people. Clin Geriatr Med 2001;17:417–31. [DOI] [PubMed] [Google Scholar]
- [24].Hoskins DL, Gordon TL, Crisp T. The effects of aging on mu and delta opioid receptors in the spinal cord of Fischer-344 rats. Brain Res 1998;791:299–302. [DOI] [PubMed] [Google Scholar]
- [25].Johnson M, Martinson M. Efficacy of electrical nerve stimulation for chronic musculoskeletal pain: a meta-analysis of randomized controlled trials. PAIN 2007;130:157–65. [DOI] [PubMed] [Google Scholar]
- [26].Kalra A, Urban MO, Sluka KA. Blockade of opioid receptors in rostral ventral medulla prevents antihyperalgesia produced by transcutaneous electrical nerve stimulation (TENS). J Pharmacol Exp Ther 2001;298:257–63. [PubMed] [Google Scholar]
- [27].Lariviere M, Goffaux P, Marchand S, Julien N. Changes in pain perception and descending inhibitory controls start at middle age in healthy adults. Clin J Pain 2007;23:506–10. [DOI] [PubMed] [Google Scholar]
- [28].Le Bars D, Dickenson AH, Besson JM. Diffuse noxious inhibitory controls (DNIC). I. Effects on dorsal horn convergent neurones in the rat. PAIN 1979;6:283–304. [DOI] [PubMed] [Google Scholar]
- [29].Leonard G, Cloutier C, Marchand S. Reduced analgesic effect of acupuncture-like TENS but not conventional TENS in opioid-treated patients. J Pain 2011;12:213–21. [DOI] [PubMed] [Google Scholar]
- [30].Leonard G, Goffaux P, Marchand S. Deciphering the role of endogenous opioids in high-frequency TENS using low and high doses of naloxone. PAIN 2010;151:215–19. [DOI] [PubMed] [Google Scholar]
- [31].Leonard G, Lafrenaye S, Goffaux P. Randomized placebo-controlled cross-over designs in clinical trials: a gold standard to be reassessed. Curr Med Res Opin 2012;28:245–8. [DOI] [PubMed] [Google Scholar]
- [32].Leonard G, Rodrigue M, Cloutier C, Marchand S. Deciphering the role of endogenous opioids in high frequency TENS using high and low doses of naloxone. IASP 12th World Congress on Pain (Glasgow) 2008. [DOI] [PubMed]
- [33].Liebano RE, Rakel B, Vance CG, Walsh DM, Sluka KA. An investigation of the development of analgesic tolerance to TENS in humans. PAIN 2011;152:335–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Mannheimer C, Carlsson CA. The analgesic effect of transcutaneous electrical nerve stimulation (TNS) in patients with rheumatoid arthritis. A comparative study of different pulse patterns. PAIN 1979;6:329–34. [DOI] [PubMed] [Google Scholar]
- [35].Marchand S. The phenomenon of pain. Seattle: IASP Press, 2012. [Google Scholar]
- [36].Marchand S. Applied pain neurophysiology. In: Beaulieu P, Lussier D, Porreca F, Dickenson AH, editors. Pharmacology of pain. Seattle: IASP Press, 2010. p. 3–26. [Google Scholar]
- [37].Marchand S, Charest J, Li J, Chenard JR, Lavignolle B, Laurencelle L. Is TENS purely a placebo effect? A controlled study on chronic low back pain. PAIN 1993;54:99–106. [DOI] [PubMed] [Google Scholar]
- [38].Marchand S, Li J, Charest J. Effects of caffeine on analgesia from transcutaneous electrical nerve stimulation. N Engl J Med 1995;333:325–6. [DOI] [PubMed] [Google Scholar]
- [39].Martin DC, Rubin FH. Anatomy and physiology of the aging brain. In The handbook of neuropsychology and aging. New York: Plenum Press, 1997. p. 32–43. [Google Scholar]
- [40].McLachlan AJ, Hilmer SN, Le Couteur DG. Variability in response to medicines in older people: phenotypic and genotypic factors. Clin Pharmacol Ther 2009;85:431–3. [DOI] [PubMed] [Google Scholar]
- [41].Melzack R, Wall PD. Pain mechanisms: a new theory. Science 1965;150:971–9. [DOI] [PubMed] [Google Scholar]
- [42].Moloney NA, Hall TM, O'Sullivan TC, Doody CM. Reliability of thermal quantitative sensory testing of the hand in a cohort of young, healthy adults. Muscle Nerve 2011;44:547–52. [DOI] [PubMed] [Google Scholar]
- [43].Naert AL, Kehlet H, Kupers R. Characterization of a novel model of tonic heat pain stimulation in healthy volunteers. PAIN 2008;138:163–71. [DOI] [PubMed] [Google Scholar]
- [44].Ng MM, Leung MC, Poon DM. The effects of electro-acupuncture and transcutaneous electrical nerve stimulation on patients with painful osteoarthritic knees: a randomized controlled trial with follow-up evaluation. J Altern Complement Med 2003;9:641–9. [DOI] [PubMed] [Google Scholar]
- [45].Pak-Woodruff DS. Normal aging and the brain. In: The neuropsychology of aging. Oxford: BlakWell Publishers, 1997. P. 86–108. [Google Scholar]
- [46].Palmer ST, Martin DJ, Steedman WM, Ravey J. Effects of electric stimulation on C and A delta fiber-mediated thermal perception thresholds. Arch Phys Med Rehabil 2004;85:119–28. [DOI] [PubMed] [Google Scholar]
- [47].Pantaleao MA, Laurino MF, Gallego NL, Cabral CM, Rakel B, Vance C, Sluka KA, Walsh DM, Liebano RE. Adjusting pulse amplitude during TENS application produces greater hypoalgesia. J Pain 2011;12:581–90. [DOI] [PubMed] [Google Scholar]
- [48].Park J, Hughes AK. Nonpharmacological approaches to the management of chronic pain in community-dwelling older adults: a review of empirical evidence. J Am Geriatr Soc 2012;60:555–68. [DOI] [PubMed] [Google Scholar]
- [49].Patel KV, Guralnik JM, Dansie EJ, Turk DC. Prevalence and impact of pain among older adults in the United States: findings from the 2011 National Health and Aging Trends Study. PAIN 2013;154:2649–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Ramage-Morin PL. Medication use among senior Canadians. Health Rep 2009;20:37–44. [PubMed] [Google Scholar]
- [51].Sjolund BH, Eriksson MB. The influence of naloxone on analgesia produced by peripheral conditioning stimulation. Brain Res 1979;173:295–301. [DOI] [PubMed] [Google Scholar]
- [52].Sluka KA, Bjordal JM, Marchand S, Rakel BA. What makes transcutaneous electrical nerve stimulation work? Making sense of the mixed results in the clinical literature. Phys Ther 2013;93:1397–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Sluka KA, Judge MA, McColley MM, Reveiz PM, Taylor BM. Low frequency TENS is less effective than high frequency TENS at reducing inflammation-induced hyperalgesia in morphine-tolerant rats. Eur J Pain 2000;4:185–93. [DOI] [PubMed] [Google Scholar]
- [54].Sluka KA, Walsh D. Transcutaneous electrical nerve stimulation: basic science mechanisms and clinical effectiveness. J Pain 2003;4:109–21. [DOI] [PubMed] [Google Scholar]
- [55].Tong KC, Lo SK, Cheing GL. Alternating frequencies of transcutaneous electric nerve stimulation: does it produce greater analgesic effects on mechanical and thermal pain thresholds? Arch Phys Med Rehabil 2007;88:1344–9. [DOI] [PubMed] [Google Scholar]
- [56].Tulgar M, Tulgar O, Herken H. Psychophysical responses to experimentally induced heat and cold pain before, during, and after transcutaneous electrical nerve stimulation. Neuromodulation 2003;6:229–36. [DOI] [PubMed] [Google Scholar]
- [57].Urwin M, Symmons D, Allison T, Brammah T, Busby H, Roxby M, Simmons A, Williams G. Estimating the burden of musculoskeletal disorders in the community: the comparative prevalence of symptoms at different anatomical sites, and the relation to social deprivation. Ann Rheum Dis 1998;57:649–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Vance CG, Dailey DL, Rakel BA, Sluka KA. Using TENS for pain control: the state of the evidence. Pain Manag 2014;4:197–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Wang N, Hui-Chan C. Effects of acupoints TENS on heat pain threshold in normal subjects. Chin Med J (Engl) 2003;116:1864–8. [PubMed] [Google Scholar]
- [60].Willer JC, Bouhassira D, Le BD. Neurophysiological bases of the counterirritation phenomenon: diffuse control inhibitors induced by nociceptive stimulation [in French]. Neurophysiol Clin 1999;29:379–400. [DOI] [PubMed] [Google Scholar]
- [61].Yarnitsky D, Sprecher E, Zaslansky R, Hemli JA. Heat pain thresholds: normative data and repeatability. PAIN 1995;60:329–32. [DOI] [PubMed] [Google Scholar]
- [62].Zakoscielna KM, Parmelee PA. Pain variability and its predictors in older adults: depression, cognition, functional status, health, and pain. J Aging Health 2013;25:1329–39. [DOI] [PubMed] [Google Scholar]


