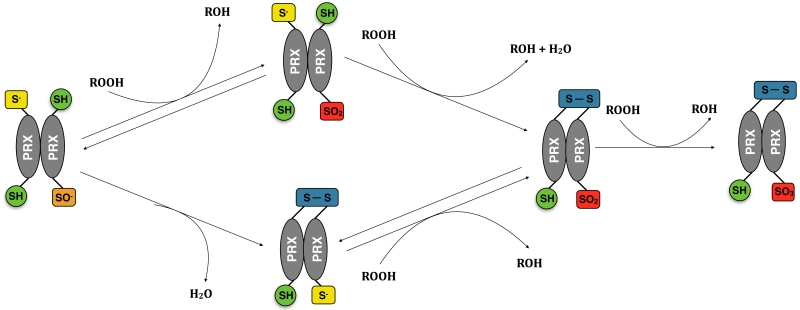
Figure 2.
Routes to formation of over-oxidised dimers. An oxidised PRX dimer could either form a disulphide bond via condensation reaction between CysP and CysR (1) or be further oxidised to sulphinic acid (2). Since a dimer possesses a second pair of CysP and CysR, it could undergo another catalytic cycle and form an over-oxidised dimer. The over-oxidized dimer could form by further oxidation of a disulfide-linked dimer (3), or by the formation of a disulfide bond between an over-oxidised monomer and a reduced monomer (4). The over-oxidised dimer could in turn be oxidised to form a sulphonic acid (5), which is regarded as irreversibly oxidised, and is recycled via proteosomal degradation. For clarity, in step 3 we have omitted the first oxidation reaction forming sulphenic acid and the reverse reaction, involving the reduction of the oxidized species by the thioredoxin system.