Figure 4.
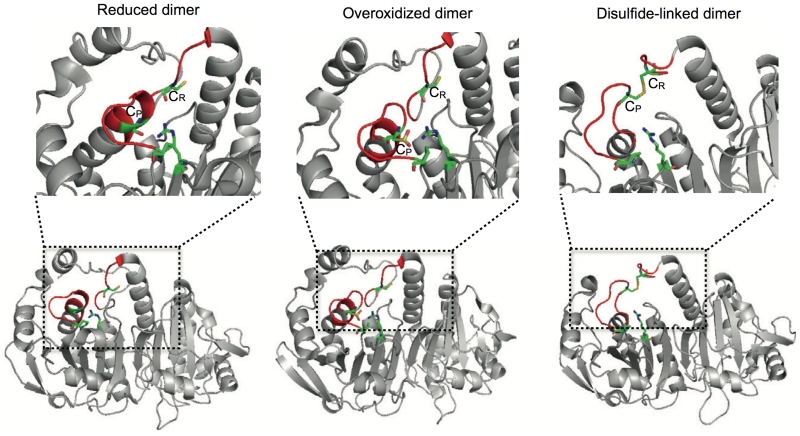
The structure of the 2-Cys PRX dimer is governed by the oxidation status of the active site cysteine. Lower panel. Reduced (PDB entry 2Z9S), over-oxidized (PDB entry 1QMV) and disulfide-linked (PDB entry 1QQ2) dimers represented in ribbon diagrams. The reduced and over-oxidized dimers assume almost identical conformations, with the active site loop (shown in red) in the fully folded state. Notice that the active site loop in the disulfide-linked dimer is partially unfolded to allow the CR-CP interaction. Top panel. The active sites of the dimers - CysP,CysR and the stabilizing Arg128 (Arg127 for 1QQ2) and Pro45 (Pro44 for 1QQ2) are shown in “sticks” representation, the regions in the active site which undergo significant structural rearrangements to allow disulphide bond formation are coloured in red.