Abstract
The G2 DNA damage checkpoint is one of the most important mechanisms controlling G2–mitosis transition. The kinase Greatwall (MASTL in human) promotes normal G2–mitosis transition by inhibiting PP2A via ARPP19 and ENSA. In this study, we demonstrate that MASTL is critical for maintaining genome integrity after DNA damage. Although MASTL did not affect the activation of DNA damage responses and subsequent repair, it determined the timing of entry into mitosis and the subsequent fate of the recovering cells. Constitutively active MASTL promoted dephosphorylation of CDK1Tyr15 and accelerated mitotic entry after DNA damage. Conversely, downregulation of MASTL or ARPP19/ENSA delayed mitotic entry. Remarkably, APC/C was activated precociously, resulting in the damaged cells progressing from G2 directly to G1 and skipping mitosis all together. Collectively, these results established that precise control of MASTL is essential to couple DNA damage to mitosis through the rate of mitotic entry and APC/C activation.
Greatwall kinase (MASTL in human) has emerged as a key player for mitosis. Greatwall/MASTL phosphorylates two small proteins called ARPP19 and α-endosulfine (ENSA), stimulating their inhibition of PP2A–B55δ1,2. As PP2A–B55δ is the major phosphatase that dephosphorylates CDK1 substrates, Greatwall/MASTL plays a pivotal role in maintaining CDK1-dependent phosphorylation during mitosis3,4,5 CDK1 activates Greatwall/MASTL during mitosis in a positive feedback loop6.
A crucial role of Greatwall/MASTL appears to be for regulating the activity of cyclin B1–CDK1. In support of this, depletion of Greatwall in Xenopus egg extracts stimulates the accumulation of Thr14/Tyr15-phosphorylated CDK16, which could in part be explained by the maintenance of CDC25 and WEE1 phosphorylation by the Greatwall/MASTL pathway7. In human cells, depletion of MASTL also induces a G2 arrest. Partial depletion of MASTL, however, induces multiple mitotic defects that include the spindle-assembly checkpoint and cytokinesis, indicating that MASTL is also important for the maintenance of cyclin B1–CDK1 activity during mitosis3. In agreement with this, conditional knockout of the mouse MASTL indicates that cells can enter mitosis with normal kinetics without MASTL, but they display mitotic collapse after nuclear envelope breakdown8.
To maintain genome integrity, it is vital for cells to halt mitotic entry after DNA damage. A surveillance mechanism termed the G2 DNA damage checkpoint monitors DNA integrity and prevents entry into mitosis9. Following DNA double-strand breaks, ATM is autophosphorylated, leading to release of active monomers from homodimer complexes. ATM then phosphorylates residues in the SQ/TQ domain of CHK1/CHK2, stimulating the activity of these effector kinases10. CHK1/CHK2 in turn activates WEE1 and represses the CDC25 phosphatase family, thereby maintaining CDK1 in an inhibitory phosphorylated state11.
Given that MASTL is now established as a key regulator of G2 and mitosis, we hypothesize that MASTL may play a role in preventing damaged cells from entering mitosis. Indeed, there is evidence from experiments using Xenopus egg extracts that Greatwall is inhibited after DNA damage and its activity is required for checkpoint recovery12. Furthermore, direct association and phosphorylation by Plx1 (PLK1 homolog in Xenopus) are important for the function of Greatwall in checkpoint recovery13. Whether this is true for DNA damage responses in the cellular context remains an important and outstanding issue. Here we found that in human cells, MASTL determines the timing of mitotic entry after DNA damage and provides a link between the G2 DNA damage checkpoint and APC/C activity.
Results
The rapid G2 DNA damage responses are not dependent on MASTL
We first used synchronized HeLa cells to verify that the abundance of MASTL remained constant during G2 and mitosis (Fig. 1A). Bacterially expressed ENSA was used as a substrate to assay the kinase activity of the MASTL. Coincided with the gel mobility shifts of MASTL (Fig. 1B), the kinase activity of MASTL from mitotic cells was higher than that of MASTL isolated from G1 or G2 (Fig. 1C).
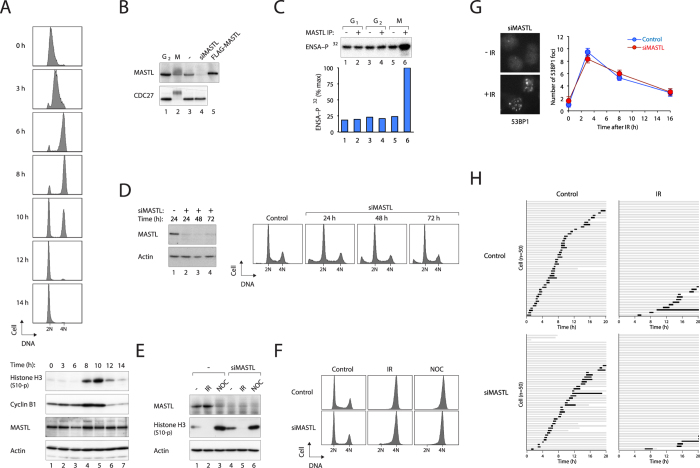
Figure 1. Activation of the G2 DNA damage checkpoint is independent on MASTL.
(A) MASTL does not vary significantly during the cell cycle. HeLa cells were synchronized using a double thymidine procedure. At different time points, the cells were harvested and analyzed with flow cytometry (upper panel) and immunoblotting (lower panel). Cyclin B1 and histone H3Ser10 phosphorylation indicated the timing of mitosis. (B) MASTL in G2 and mitosis. Lysates from G2 and mitosis (M) were analyzed with immunoblotting. MASTL was extensively phosphorylated during mitosis. Cells transfected with MASTL siRNA (siMASTL) or plasmids expressing FLAG-MASTL were loaded to validate the identity of the MASTL band. (C) Low kinase activity of MASTL during interphase. Different lysates were subjected to immunoprecipitation using either control or MASTL antiserum. The kinase activity was assayed using purified 6xHis-ENSA as a substrate. (D) Downregulation of MASTL does not affect unperturbed cell cycle distribution. Transfected HeLa cells were harvested at the indicated time points and analyzed. (E) IR-mediated interphase arrest is independent on MASTL. HeLa cells were transfected as indicated. After 48 h, the cells were treated with IR (15 Gy) or incubated with nocodazole (NOC). After another 16 h, the cells were harvested for immunoblotting. (F) IR-induced G2 arrest is independent on MASTL. HeLa cells transfected as indicated were irradiated (15 Gy). After another 16 h, the cells were analyzed with flow cytometry. NOC treatment (16 h) was used as a mitotic control. (G) MASTL does not affect IR-mediated 53BP1 foci formation or repair. HeLa cells transfected as indicated were irradiated (2 Gy) and fixed at different time points. The number of 53BP1 foci per cell was quantified (n = 100; mean ± 95% CI). Examples of 53BP1 staining of siMASTL-transfected cells in the presence and absence of IR treatment (3 h) are shown. (H) Mitosis is inhibited by IR in the absence of MASTL. HeLa cells expressing histone H2B-GFP were transfected as indicated. After 48 h, the cells were either mock-treated or irradiated (15 Gy). Individual cells were then tracked with time-lapse microscopy. Each horizontal bar represents one cell (n = 50). Grey: interphase; black: mitosis (from DNA condensation to anaphase); truncated bars: cell death.
As MASTL is normally activated during mitosis, we hypothesized that keeping MASTL inactive is important for the proper function of the G2 DNA damage checkpoint. To test this hypothesis, we initially examined if the activation of the checkpoint is compromised when MASTL was either overexpressed or depleted. HeLa cells were used as a model in this study because IR exclusively activated the G2 DNA damage checkpoint in these cells (p53 is degraded by HPV E6).
Downregulation of MASTL with siRNA (siMASTL) did not affect unperturbed cell cycle distribution (Fig. 1D). Figure 1E shows that the expression of MASTL was not altered by ionizing radiation (IR)-mediated DNA damage. Inhibition of mitotic entry (which is reflected by the absence of histone H3Ser10 phosphorylation) was not affected by MASTL depletion. Both control and MASTL-depleted cells were arrested with G2/M DNA contents at 16 hours after irradiation (Fig. 1F). Expression of siMASTL also did not alter the number of IR-induced 53BP1 foci, indicating that the early responses to double strand breaks were unaffected by MASTL (Fig. 1G). We further validated the presence of G2 DNA damage responses by using live-cell imaging to follow individual cells after DNA damage. Figure 1H shows that although transfection of siMASTL extended the interphase in the unperturbed cell cycle, it did not abolish mitotic entry. Treatment with IR inhibited mitotic entry in both control and siMASTL-transfected cells, indicating that downregulation of MASTL did not compromise the DNA damage-mediated G2 delay.
To determine if overexpression of MASTL affects the DNA damage responses, we generated cell lines expressing MASTL under the control of doxycycline (Dox). The recombinant MASTL could be distinguished from the endogenous MASTL by the change in size caused by the FLAG and EGFP tags. The FLAG-EGFP-MASTL was significantly overexpressed (~50×) compared to the endogenous protein (Fig. 2A). Neither the IR-mediated 53BP1 foci formation (Supplemental Fig. S1A) nor the G2 arrest (Fig. 2B) was affected by the ectopically expressed MASTL.
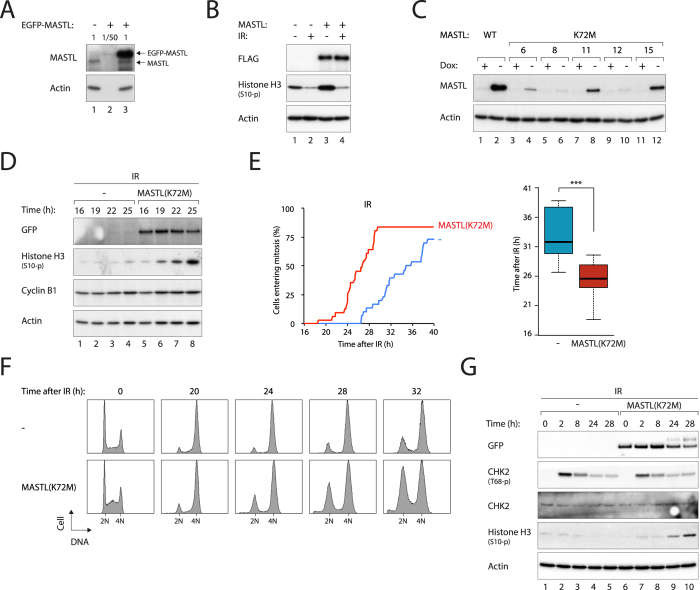
Figure 2. DNA damage-induced G2 arrest can be interrupted by MASTL.
(A) Ectopic expression of MASTL. A HeLa cell line expressing FLAG-EGFP-MASTL was generated. Lysates were prepared and different dilutions were loaded to compare with normal HeLa cells. (B) Overexpression of MASTL does not affect checkpoint activation. HeLa cells expressing FLAG-EGFP-MASTL were irradiated (15 Gy). NOC was added to trap cells in mitosis. After 6 h, the cells were harvested and analyzed. The expression of exogenous MASTL was detected with antibodies against FLAG. (C) Expression of MASTLK72M. Cell lines expressing FLAG-EGFP-MASTLK72M under the control of doxycycline (Dox) were generated. MASTLK72M expression in different clones was compared to the cell line expressing wild type MASTL. Clone#15 was used in other experiments in this paper. (D) MASTLK72M promotes mitotic entry after DNA damage. Cells were grown in the absence or presence of Dox for 48 h to turn on or off MASTLK72M respectively before treated with IR (15 Gy). After 16 h, the cells were incubated with NOC and harvested at different time points for immunoblotting. (E) MASTLK72M accelerates mitotic entry after DNA damage. Cells were grown in the absence or presence of Dox for 48 h. The cells were then transfected with a plasmid expressing histone H2B-mRFP. After 24 h, the cells were treated with IR (15 Gy). After another 16 h, individual cells were tracked with time-lapse microscopy to analyze the time of mitotic entry (n = 50). The median mitotic entry time was plotted as a box-and-whisker chart. Expression of MASTLK72M significantly accelerated entry into mitosis (P < 0.001). (F) MASTLK72M shortens G2 arrest after DNA damage. Cells were grown in the absence or presence of Dox for 48 h. After treating with IR (15 Gy), the cells were harvested at the indicated time points and analyzed with flow cytometry. (G) MASTLK72M does not affect inactivation of the checkpoint. Cells were grown in the absence or presence of Dox for 48 h. After treating with IR (15 Gy) and NOC, the cells were harvested at the indicated time points. The expression of the indicated proteins was detected with immunoblotting.
These experiments indicate that depletion or overexpression of MASTL does not perturb the rapid DNA damage responses or the subsequent G2 cell cycle arrest and DNA repair.
MASTL controls the timing of mitotic entry after DNA damage
DNA damaged cells can continue the cell cycle and enter mitosis after the DNA is repaired. Alternatively, the cells can enter mitosis even when the DNA is not fully repaired by a process involving adaptation. While cells could repair most of the DNA damage after relatively low dose of IR (2 Gy) (Fig. S1), mitotic entry after higher dose of IR (15 Gy) was mainly due to adaptation (based on the presence of 53BP1 foci at the time of mitotic entry, data not shown). Both cases (as well as forced checkpoint abrogation below) were defined as checkpoint recovery here, which were characterized by mitotic entry and accumulation of histone H3Ser10 phosphorylation.
We found that overexpression of MASTL marginally accelerated mitotic entry after DNA damage (Fig. S2A). To overcome the possibility that the expressed MASTL remained inactive, cells expressing a hyperactive mutant of MASTL were generated (changing the lysine at residue 72 to methionine14,15). Although MASTLK72M was expressed to a level lower than wild type MASTL (Fig. 2C; which is consistent with data from Drosophila and Xenopus14,15), it strongly stimulated mitotic entry after DNA damage (Fig. 2D).
Time-lapse imaging provided further evidence that MASTLK72M promoted mitotic entry after DNA damage. While irradiated control cells (grown in the presence of Dox) started to enter mitosis after a delay of 25 hours, MASTLK72M-expressing cells entered mitosis significantly earlier, reaching ~50% by 25 hours (Fig. 2E). This was further validated using flow cytometry (Fig. 2F). Both control and MASTLK72M-expressing cells were arrested with G2/M DNA contents at 20 hours after IR treatment. While the majority of control cells continued to arrest over the next 12 hours, up to half of the MASTLK72M-expressing cells recovered and entered G1. The different recovery time was not due to different levels of initial DNA damage, because similar number of 53BP1 foci was induced by IR in the presence or absence of MASTLK72M (Fig. S1B). MASTLK72M also did not affect the inactivation of the checkpoint, as CHK2 was inactivated (as indicated by Thr68 dephosphorylation) at the same rate in the presence or absence of MASTLK72M (Fig. 2G).
As mitotic entry after irradiation occurred only after a delay of over 24 hours, chemical inhibitors of the checkpoint were also used to mimic checkpoint recovery16 (Fig. 3B). As anticipated, incubation of IR-arrested cells with the CHK1 inhibitor AZD7762 promoted mitosis (as detected by histone H3Ser10 phosphorylation). This was speeded up after overexpression of either MASTLK72M (Fig. 3B) or MASTL (Fig. S2B). Mitotic entry after AZD7762 treatment was also accelerated by MASTLK72M (Fig. 3C) or MASTL (Fig. S2C). Mechanistically, the accelerated checkpoint recovery was correlated with precocious dephosphorylation of CDK1Tyr15, suggesting that MASTL promoted mitotic entry after DNA damage through regulating CDK1 inhibitory phosphorylation (Fig. 3B).
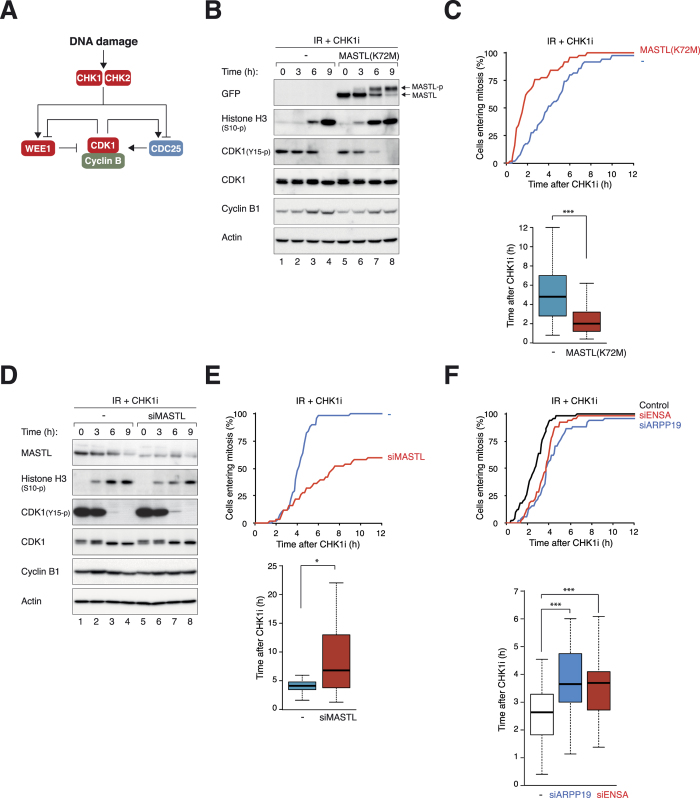
Figure 3. MASTL controls the timing of mitotic entry after DNA damage.
(A) Inhibition of CHK1/CHK2 bypasses the G2 DNA damage checkpoint. DNA damage activates CHK1/CHK2. This results in the activation of WEE1 and inhibition of CDC25, thereby keeping CDK1 in an inhibitory phosphorylated state. Inhibitors of CHK1/CHK2 induce damaged cells to enter mitosis. (B) MASTLK72M promotes mitotic entry after AZD7762-mediated checkpoint abrogation. HeLa cells expressing FLAG-EGFP-MASTLK72M were grown in the presence or absence of Dox(48 h) before irradiated (15 Gy). After 16 h, the cells were incubated with AZD7762 (CHK1i) and NOC (to trap cells in mitosis) and harvested at different time points. Phosphorylation of MASTL during mitosis induced a gel mobility shift (MASTL-p). (C) MASTLK72M accelerates mitotic entry after checkpoint abrogation. HeLa cells expressing FLAG-EGFP-MASTLK72M were grown with or without Dox(48 h) before transfected with histone H2B-mRFP-expressing plasmids. After 24 h, the cells were irradiated (15 Gy). After another 16 h, the cells were incubated with CHK1i. Individual cells were then tracked with time-lapse microscopy to analyze the time of mitotic entry (n = 50)(upper panel). The median mitotic entry time was plotted as a box-and-whisker chart (lower panel). MASTLK72M significantly accelerated mitotic entry (P < 0.001). (D) Depletion of MASTL delays mitotic entry after DNA damage. HeLa cells were transfected with either control or siMASTL for 48 h before irradiated (15 Gy). After 16 h, the cells were incubated with CHK1i and NOC and harvested at different time points. (E) Depletion of MASTL delays mitotic entry after checkpoint abrogation. HeLa cells expressing histone H2B-GFP were transfected with either control or siMASTL for 48 h before irradiated (15 Gy). After another 16 h, the cells were incubated with CHK1i. Individual cells were then analyzed with time-lapse microscopy as in panel (C). Depletion of MASTL significantly delayed entry into mitosis (P < 0.05). (F) Depletion of ARPP19 or ENSA delays mitotic entry after checkpoint abrogation. HeLa cells expressing histone H2B-GFP were transfected with control, siARPP19, or siENSA for 48 h before irradiated (15 Gy). After another 16 h, the cells were incubated with CHK1i and analyzed with time-lapse microscopy as in panel (C). Depletion of ARPP19 or ENSA significantly delayed entry into mitosis (P < 0.001).
We further performed the converse experiments to determine if silencing of MASTL delayed mitotic entry after DNA damage. Figure 3D shows that the AZD7762-induced histone H3Ser10 phosphorylation was delayed after MASTL depletion. Live-cell imaging confirmed that mitotic entry after checkpoint abrogation was delayed after MASTL depletion (Fig. 3E). Similar effects on checkpoint recovery by siMASTL were obtained using another CHK1 inhibitor (UCN-01) (Fig. S3A,B).
ENSA and ARPP19 are two key downstream substrates of MASTL17. To test if the effects of siMASTL were specific for the MASTL pathway, we next examined if downregulation of ENSA or ARPP19 could induce similar effects as siMASTL. Immunoblotting showed that ENSA could be depleted with siRNAs (Fig. S4A). As ENSA and ARPP19 share >70% amino acid identity and both proteins were recognized by the antibodies (revealed by using recombinant ENSA and ARPP19, data not shown), our results suggested that ENSA was probably more abundant than ARPP19, and that siARPP19 could further deplete the ENSA/ARPP19 band after ENSA was depleted (Fig. S4A, compare lanes 2 and 6). Although no specific antibodies against ARPP19 were available, siARPP19 could at least effectively downregulate recombinant ARPP19 (Fig. S4B). Moreover, siARPP19 did not affect the level of endogenous or transfected ENSA (Fig. S4A,B).
Consistent with the results using siMASTL, we found that mitotic entry after DNA damage was delayed after silencing of either ARPP19 or ENSA (Fig. 3F). Experiments with siARPP19 and siENSA together were not performed because depletion of both proteins induced substantial cell cycle defects (Fig. S4C) and inhibited entry into mitosis (Fig. S4D).
Collectively, these results indicate that mitotic entry after recovery from the G2 DNA damage checkpoint is accelerated by active MASTL and delayed in the absence of MASTL.
MASTL prevents premature activation of APC/C after DNA damage
We next examined if spontaneous checkpoint recovery is also affected by MASTL depletion. As shown above, MASTL-depleted cells could be arrested in G2 at 16 hours after irradiation (Fig. 1F). Further incubation (48 h) resulted in genome re-duplication in siMASTL-transfected but not in control cells (Fig. 4A). By contrast, genome re-duplication induced by pharmacological inhibition of CDK118 occurred in both control and MASTL-depleted cells. Because substantial apoptosis occurred at 48 hours after DNA damage, we also performed the same experiment in the presence of a pan-caspase inhibitor. Figure 4B shows more unambiguously that genome re-duplication occurred in IR-treated siMASTL-transfected cells. These results suggested that in the absence of MASTL, DNA-damaged cells could not sustain a prolonged G2 arrest and undergo genome re-duplication. Genome re-duplication of IR-treated cells was also promoted by siARPP19 (but not siENSA), albeit less robustly than siMASTL (Fig. 4C). These results support the hypothesis that the MASTL pathway is required to sustain the DNA damage-mediated G2 arrest.
Figure 4. MASTL is required to prevent endoreplication after DNA damage.
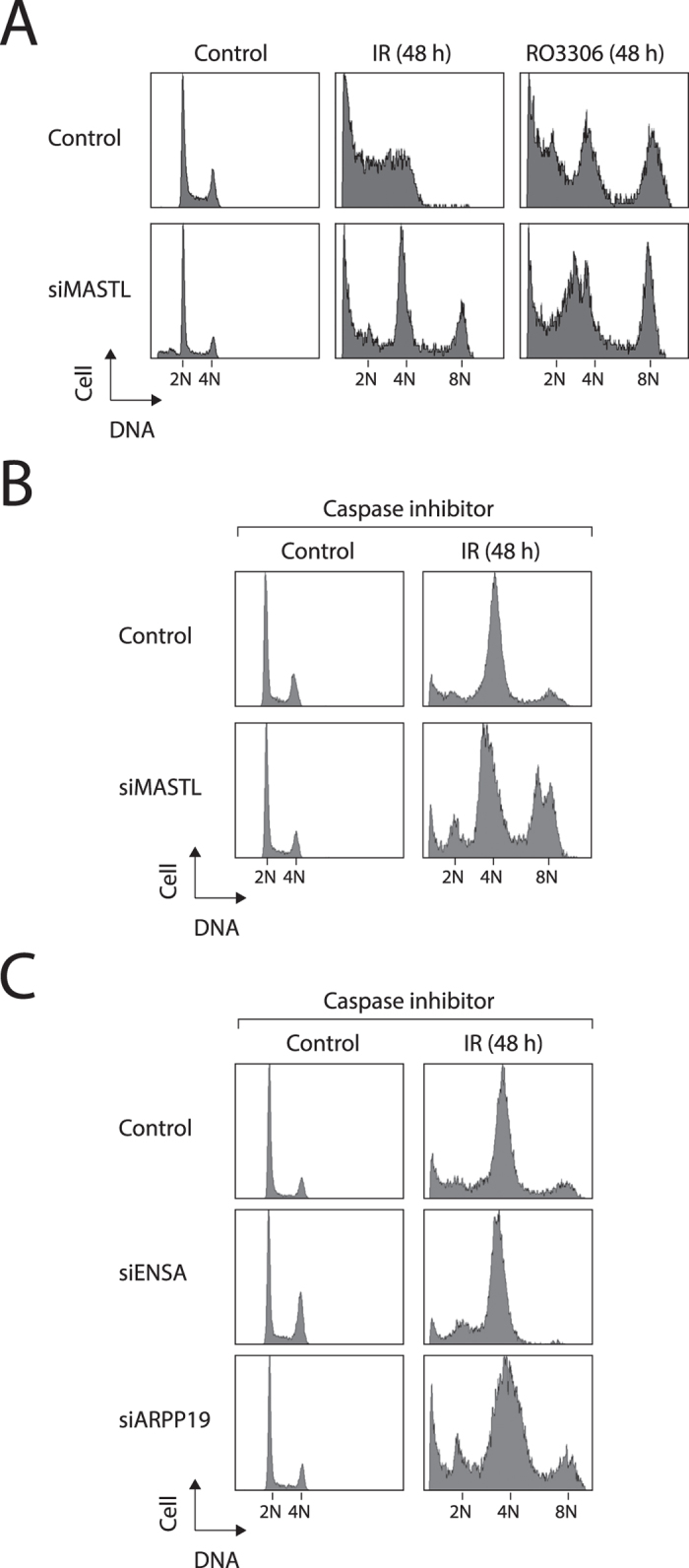
(A) Depletion of MASTL induces DNA re-replication after DNA damage. HeLa cells were transfected with either control or siMASTL for 48 h. The cells were then either irradiated (15 Gy) or incubated with the CDK1 inhibitor RO3306 (CDK1i). After another 48 h, the cells were harvested and analyzed with flow cytometry. The present of cells containing 8N DNA contents indicates endoreplication. (B) Depletion of MASTL induces DNA re-replication after DNA damage. HeLa cells were transfected with either control or siMASTL for 48 h. The cells were then irradiated and incubated with a pan-caspase inhibitor. After another 48 h, the cells were harvested and analyzed with flow cytometry. (C) Depletion of ARPP19 induces DNA re-replication after DNA damage. HeLa cells were transfected with control, siENSA, or siARPP19 for 48 h. The cells were then irradiated (15 Gy) and incubated with a pan-caspase inhibitor. After another 48 h, the cells were harvested and analyzed with flow cytometry.
To understand how the cell cycle of MASTL-depleted cells is regulated after DNA damage, cells expressing the FUCCI (Fluorescent Ubiquitin-based Cell Cycle Indicators) cell cycle reporter system were analyzed. The system consists of an APC/C target (mVenus-Geminin, present from early S phase to the end of mitosis) and a SCF complex target (mCherry-CDT1, present from early G1 phase to the beginning of S phase)19. Instead of using caspase inhibitors to overcome the apoptosis caused by DNA damage, we engineered a cell line to express both FUCCI and the anti-apoptotic protein BCL2. Surprisingly, MASTL-depleted cells did not enter mitosis when they recover from DNA damage. Cells in S/G2 (expressing mVenus-Geminin) switched to G1 without going through mitosis. Frequently cells underwent multiple rounds of S/G2–G1 switches within the imaging period (an example is shown in Fig. 5A, Video 1). By contrast, control cells that were in S/G2 at the time of irradiation continued to express mVenus-Geminin until checkpoint recovery, when they underwent mitosis (Fig. 5A, Video 2).
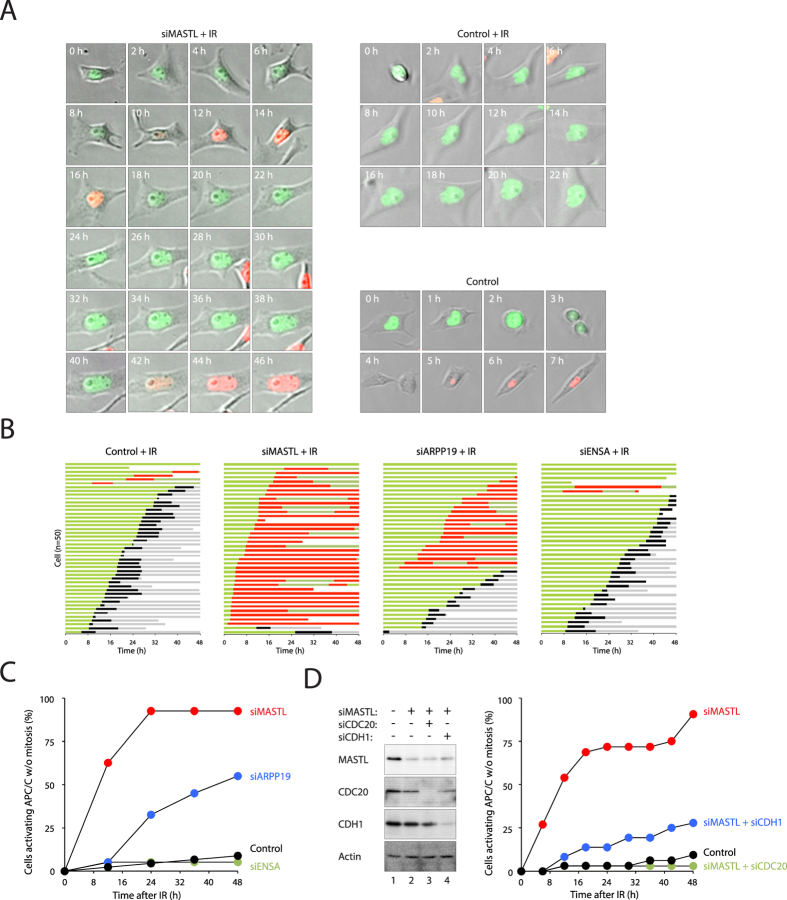
Figure 5. Depletion of MASTL induces premature activation of APC/C after DNA damage.
(A) MASTL-depleted cells switch from G2 to G1 directly after DNA damage. HeLa cells expressing FUCCI and BCL-2 were transfected with either control or siMASTL. After 48 h, the cells were treated with IR (15 Gy) and analyzed with time-lapse microscopy. An example of MASTL-depleted cells switching between states that expressed the S/G2 reporter (mVenus-Geminin, green) and the G1 reporter (mCherry-CDT1, red) without an intervening mitosis is shown (two cycles of oscillation in this example). Examples of control-transfected cells that continued to express the S/G2 reporter after IR treatment, and a cell without IR are also shown. (B) Depletion of either MASTL or ARPP19 can induce the G2–G1 switch. HeLa cells expressing FUCCI and BCL-2 were transfected with the indicated siRNAs. After 48 h, the cells were treated with IR (15 Gy). Cells expressing the S/G2 reporter at the time of irradiation were then tracked for 48 h with time-lapse microscopy. Each horizontal bar represents one cell (n = 50). Green: S/G2 (expressing mVenus-Geminin); red: G1 (expressing mCherry-CDT1); black: mitosis (from DNA condensation to anaphase); grey: interphase after normal mitosis; truncated bars: cell death. (C) Depletion of MASTL or ARPP19 induces premature activation of APC/C after DNA damage. The experiment was performed as described in panel (B). The percentages of cells expressing the S/G2 reporter that prematurely activated the APC/C without mitosis are quantified (n = 50). (D) Co-depletion of CDC20 or CDH1 abolishes the premature activation of APC/C in MASTL-depleted cells. HeLa cells expressing FUCCI and BCL-2 were transfected with the indicated siRNAs. After 48 h, the cells were treated with IR (15 Gy). Cells expressing the S/G2 reporter at the time of irradiation were then tracked for 48 h with time-lapse microscopy. The percentages of cells expressing the S/G2 reporter that prematurely activated the APC/C without mitosis are quantified (n = 50). Lysates were also prepared from cells before the imaging and subjected to immunoblotting analysis to verify the depletion of the indicated proteins.
We found that while the majority of control irradiated cells eventually entered mitosis, >95% of irradiated MASTL-depleted cells activated APC/C prematurely without mitosis (Fig. 5B,C). Cells that were in G1 phase at the time of irradiation behaved similarly, except that they first entered S/G2 phase before degrading the APC/C reporter (data not shown). The irradiated MASTL-depleted cells did not display any morphological signs associated with mitosis. This was in contrast to FUCCI-expressing cells that underwent normal mitosis (Fig. 5A, Video 3). Using MASTL-depleted cells expressing histone H2B-GFP instead of FUCCI, we confirmed that there was no DNA condensation to suggest even a very brief mitotic state before APC/C was activated (data not shown).
Similar results were also obtained in U2OS cells expressing the FUCCI reporters (Fig. S5), indicating that the effects of siMASTL on APC/C after DNA damage were not limited to one cell type.
To validate these results, we also used a second siRNA to target MASTL. Although both siRNAs could robustly inhibit the expression of transfected FLAG-MASTL (Fig. S6A), the second siMASTL was less effective in downregulating endogenous MASTL (Fig. S6B). Nevertheless, the second siMASTL also induced prematurely APC/C activation after DNA damage in a large portion of cells (Fig. S6C). We also found that prematurely APC/C activation also occurred after siMASTL-transfected cells were irradiated with lower doses of IR (Fig. S6D).
To further validate the importance of MASTL pathway in preventing premature APC/C activation after DNA damage, we also performed similar experiments using siENSA and siARPP19. Figure 5C shows that siARPP19 (but not siENSA) also promoted premature APC/C activation after DNA damage.
Finally, we verified that the premature degardation of the S/G2 reporter in MASTL-depleted cells was indeed APC/C-dependent by targeting critical APC/C components. Cells were transfected with siMASTL either alone or together with siRNAs against CDC20 or CDH1 (two critical targeting subunits of APC/C). The premature activation of APC/C caused by siMASTL was inhibited after co-depletion of either CDC20 or CDH1 (Fig. 5D).
In conclusion, these results indicate that the MASTL pathway is required for DNA-damaged cells to maintain a proper G2 arrest; loss of the pathway results in delayed mitotic entry, premature APC/C activation, and endoreplication.
Discussion
In this study, we provide evidence that MASTL is critical for G2 DNA damage responses. Our conclusion is that although activation and inactivation of the DNA damage checkpoint was not affected by MASTL, the speed of mitotic entry after DNA damage and cell fate were determined by the expression of MASTL. While overexpression of MASTL accelerated mitotic entry, downregulation of MASTL delayed mitotic entry after DNA damage. In addition to delaying mitotic entry, downregulation of MASTL also resulted in premature activation of APC/C. Hence the genome instability induced by MASTL downregulation was associated with genome reduplication.
Our available evidence indicated that DNA damage could initially activate the G2 DNA damage checkpoint with or without MASTL. For example, 53BP1 foci formation at damaged sites appeared to be normal in the presence of constitutively active MASTL (Fig. S1) or siMASTL (Fig. 1G). The initial G2 cell cycle arrest was also independent on MASTL: flow cytometry analysis (Fig. 1F), live-cell imaging (Fig. 1H), and histone H3Ser10 phosphorylation (Fig. 1E) all indicated that IR could inhibit mitosis in the absence of MASTL. Similar assays also indicated that IR inhibited mitosis in the presence of constitutively active MASTL (Fig. 2).
Cells irradiated with 15 Gy of IR were able to remain in G2 for at least 20 hours before recovery, most likely by adaptation (Fig. 2E). Several methods were employed to measure checkpoint recovery, including the re-accumulation of histone H3Ser10 phosphorylation (Fig. 2D), mitotic entry (Fig. 2E), and progression into G1 phase (Fig. 2F). They revealed that mitotic entry after DNA damage was accelerated by MASTLK72M (Figs 2D–F and 3A,B) and delayed by siMASTL (Fig. 3D,E). Likewise, mitotic entry following irradiation with a lower dose of IR (2 Gy), which was likely to occur after DNA repair, was also dependent on MASTL (data not shown).
As the kinetics of 53BP1 foci reduction was not affected by MASTL or siMASTL (Figs 1 and S1), it is unlikely that MASTL influenced the rate of DNA repair. Furthermore, mitosis following checkpoint abrogation with a CHK1 inhibitor was also accelerated by MASTL and delayed by siMASTL (Fig. 3), indicating that the effects of MASTL on mitotic entry was independent on DNA repair.
As expected, acceleration of mitotic entry after DNA damage by MASTLK72M was accompanied with an increase of mitotic defects including lagging chromosomes and bridges during anaphase. Cells with more than one observable lagging chromosomes or bridges increased from ~50% in control irradiated cells to ~80% in MASTLK72M-expressing cells (data not shown). Moreover, the MASTLK72M-expressing cells generally displayed large number of lagging chromosomes and bridges that could not be easily quantified.
The effects of MASTL on checkpoint recovery were probably due to its regulation of the kinetics of CDK1 activation and re-entry into the cell cycle. At least in Xenopus egg extracts, MASTL/Greatwall controls inhibitory phosphorylation of CDK1 through CDC25 and WEE1 during G2–mitosis7. It is possible that human MASTL also regulates CDK1 by promoting the activation of CDC25 and inactivation of WEE1 during checkpoint recovery. In support of this, MASTLK72M did not affect CHK2 inactivation (Fig. 2G) but reduced CDK1Tyr15 phosphorylation during checkpoint recovery (Fig. 3B). In contrast, the unperturbed cell cycle of MASTLK72M-expressing cells was not significantly shortened compare to that of control cells (data not shown), suggesting that MASTL may be particularly important for cell cycle reentry after DNA damage, when all the cyclin B–CDK1 complexes are in the inhibitory state.
Consistent with the results with MASTL, mitotic entry after DNA damage was delayed after silencing of either ARPP19 or ENSA (Fig. 3F). Depletion of ARPP19 and ENSA together was cytotoxic (Fig. S4). We believe that it was because siARPP19 and siENSA were more efficient than siMASTL. More complete depletion of MASTL is likely to be cytotoxic and unsuited for investigating the DNA damage checkpoint. Development of specific MASTL inhibitors should assist the study of this pathway in the future.
In addition to delaying checkpoint recovery, depletion of MASTL also triggered premature activation of APC/CCDC20 during the G2 arrest (Fig. 5). This has a decisive effect on genome integrity because degradation of cyclin B triggered G1 entry directly, leading to whole genome reduplication (Fig. 4A). MASTL acts on two similar proteins ARPP19 and ENSA. Interestingly, depletion of ARPP19 (but not ENSA) promoted premature APC/C activation (Fig. 5C) and genome rereplication (Fig. 4C) after DNA damage. These results suggest that the MASTL—ARPP19—PP2A pathway may be involved in suppressing APC/C activity after DNA damage. One possibility is that APC/C was spontaneously turned on independently on MASTL after prolonged G2 arrest. This does not occur normally because checkpoint recovery occurred before APC/C was activated. When mitotic entry was delayed, as in the case after MASTL depletion, APC/C could be activated independently on MASTL. This is similar to the premature APC/C activation when cells are arrested in G2 for a long period of time with the CDK1-specific inhibitor RO330618. However, in contrast to after DNA damage, genome re-duplication induced by pharmacological inhibition of CDK1 occurred in both control and MASTL-depleted cells (Fig. 4A), indicating that the two processes are not identical.
Another possibility is that the MASTL pathway affects APC/C activity directly. However, it is not straightforward to reconcile why depletion of either CDC20 or CDH1 abolished premature APC/C activity. APC/CCDC20 is normally activated by cyclin B–CDK1 during mitosis20, thereby preparing for the degradation of cyclin B at the end of mitosis. Before that, APC/CCDC20 is suppressed by the spindle-assembly checkpoint until all the chromosomes have achieved correct bipolar attachment. By contrast, APC/CCDH1 activity is suppressed by CDK1-dependent phosphorylation during mitosis. Hence it is not immediately obvious how a phosphatase pathway could regulate both APC/CCDC20 and APC/CCDH1 equally.
A protein called EMI1 normally suppresses APC/C activity during G2 and is removed before mitosis by a PLK1- and SCFβTrCP-dependent degradation. One possibility is that siMASTL induced EMI1 degradation during the IR-mediated arrest, thereby allowing premature APC/C activation. However, we do not favor this idea because EMI1 expression remained unchanged after MASTL was depleted (our published data). Moreover, as EMI1 degradation involves PLK1-dependent phosphorylation, we do not expect siMASTL (which increased PP2A activity) to promote this pathway.
Collectively, these findings highlight the importance of MASTL in maintaining genome stability during DNA damage responses by providing a link between the DNA damage checkpoint and mitotic regulation. On the one hand, it is paramount to keep MASTL inactive during the G2 DNA damage checkpoint. Untimely activation of MASTL induced unscheduled mitotic entry (Fig. 2D,E). On the other hand, inadequate activity of MASTL during checkpoint recovery delayed entry into mitosis as well as promoted premature APC/C activation. In this connection, it is intriguing that the level of MASTL differs considerably between different cancer cell lines (our unpublished data). It will be interesting to expand our limited understanding of whether MASTL plays a role in tumorigenesis. Another implication of this study is on the development of MASTL inhibitors, which should be able to delay mitotic entry after DNA damage and sensitize cancer cells to radiotherapies. Indeed, a recent genome-wide siRNA screen revealed that downregulation of MASTL can cause radiosensitization in non-small cell lung cancer cell lines, further supporting MASTL as a promising and druggable target21.
Methods
DNA constructs
Human MASTL cDNA in pCMV-SPORT6 (IMAGE ID: 3449913) was amplified with PCR using oligonucleotides 5′-CGCCATGGACCCCACCGCGGGAAG-3′ and 5′-CACTCGAGATGAAGGTGTGGGATT-3′; the product was cut with Nco I-Xho I and ligated into modified pUHD-P318 lacking one Xho I site to obtain FLAG-MASTLC∆310 in pUHD-P3. The Xho I-BamH I fragment from MASTL in pCMV-SPORT6 was ligated into the above MASTLC∆310 construct to obtain FLAG-MASTL in pUHD-P3. The Nco I-BamH I fragment from FLAG-MASTLC∆310 in pUHD-P3 was ligated into pUHD-P3G to obtain FLAG-EGFP-MASTLC∆310 in pUHD-P3G. A Xba I-BamH I fragment from FLAG-MASTL in pUHD-P3 was ligated into above plasmid to obtain FLAG-EGFP-MASTL in pUHD-P3G. The pUHD-P3G vector was generated as follows: the EGFP fragment from pEGFP-N3 (Clontech, Palo Alto, CA, USA) was amplified with PCR using a vector forward primer and 5′-GGCCCATGGACTTGTACAGCTCGTC-3′; the PCR product was cut with Nco I and ligated into pUHD-P318. The Nco I site upstream of EGFP was destroyed using Quikchange mutagenesis kit (Stratagene, La Jolla, CA, USA) with the primers 5′-GTTTCAGGGGCCGATGGTGAGCAA-3′ and its complement. A puromycin-resistant cassette was inserted as described into the Hind III site to generate pUHD-P3G. Site-directed mutagenesis of MASTLK72M was carried out by Quikchange mutagenesis kit using the oligonucleotides: 5′-TCAACATGAATATGACTCATCAGGT-3′ and 5′-CATATTCATGTTGATCATGTCTGCTT-3′. The Nco I-Xho I fragment from FLAG-MASTLC∆310 in pUHD-P3 was ligated into pGEG-KG to obtain GST-MASTLC∆310 in pGEX-KG. FUCCI plasmids (mVenus-Geminin and mCherry-CDT1) were gifts from Atsushi Miyawaki (RIKEN Btain Science Institute, Japan). Xenopus 6xHis-ENSA construct was a gift from Satoru Mochida (Kumamoto University, Japan).
RNA interference
A siRNA targeting MASTL (CCAUUGAGGAAUUCAGCAU, siMASTL#1) was generated by Genepharma (Shanghai, China). A second siRNA against MASTL (GGGACUUGAAACCGGACAA, siMASTL#2) was generated by RiboBio (Guangzhou, China). Two siRNAs targeting ENSA (siENSA#1: UAGCUUUGCCUCUUCAGCUCUCUCA and siENSA#2: GCAGAGCUAGUUGAGAACUCAACAU) and two siRNAs targeting ARPP19 (siARPP19#1: CCUGGAGGUUCAGAUUUCUUAAGGA and siARPP19#2: GAAGAACAAGCAACUUCCUACUGCA) were obtained from Life Technologies (Carlsbad, CA, USA). Unless stated otherwise, siRNA#1 were used in all cases. Cells were transfected with siRNA (15 nM) using LipofectamineTM RNAiMAX (Life Technologies).
Cell culture
The HeLa used in this study was a clone that expressed the tTA tetracycline transactivator22. U2OS Tet-On cell line was obtained from Clontech (Palo Alto, CA, USA). No authentication was done by the authors. Cells were propagated in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% (v/v) calf serum (HeLa) or fetal bovine serum (U2OS) and 50 U/ml penicillin-streptomycin (Life Technologies) in a humidified incubator at 37 °C in 5% CO2.
HeLa cells stably expressing histone H2B-GFP were generated as described previously23. HeLa cells expressing FLAG-EGFP-MASTL (or K72M) were generated by transfecting cells with FLAG-EGFP-MASTL (or K72M) in pUHD-P3G followed by selection with puromycin. Single colonies were isolated after about two weeks of selection.
HeLa and U2OS expressing FUCCI were generated by transfection of the FUCCI plasmids followed by cell sorting (FACSAria III, Becton Dickinson, Franklin Lakes, NJ, USA) using 488 nm and 561 nm lasers for excitation. The cells were propagated for one week before being sorted again. Three rounds of sorting were performed. Single cells-derived colonies were obtained by limited dilution of the cells into 96-well plates. The stably expression of BCL2 in the FUCCI/HeLa cells was described previously24.
Unless stated specifically, cells were treated with the following reagents at the indicated final concentration: nocodazole (Sigma-Aldrich, St. Louis, MO, USA; 0.1 μg/ml), puromycin (Sigma-Aldrich; 30 μg/ml), AZD7762 (Selleck Chemicals Houston, TX, USA; 20 nM), UCN-01 (Sigma-Aldrich; 100 nM), RO3306 (Santa Cruz Biotechnology, Santa Cruz, CA, USA; 10 μM), Z-VAD-FMK (caspase inhibitor) (Enzo Life Sciences, Farmingdale, NY, USA; 20 μM), doxycycline hydrochloride (Dox) (Sigma-Aldrich; 2 μg/ml). Cell-free extracts were prepared as described previously25. Cell cycle synchronization was performed as described26. Briefly, G2 cells were obtained at 6 h after released from a double thymidine method; mitotic cells were obtained by incubating cells released from a double thymidine method with nocodazole before the mitotic cells were isolated with mechanical shake-off; G1 cells were obtained after releasing cells from a nocodazole block.
Flow cytometry
Flow cytometry analysis after propidium iodide staining was performed as described previously27.
Ionizing radiation
IR was delivered with a caesium-137 source from a MDS Nordion (Ottawa, Canada) Gammacell 1000 Elite Irradiator.
Live-cell imaging
Cells were seeded onto a 24- or 96-well culture plates and imaged using a Ti-E-PFS inverted fluorescence microscope (Nikon, Tokyo, Japan) equipped with an ultra-low noise sCMOS camera (Andor Technology, Belfast, UK) and a TC temperature, humidity, and CO2 control system (Chamlide, Live Cell Instrument, Seoul, Korea). Data acquisition was carried out at 5 min/frame for 24 hours or 10 min/frame for 48 hours. Note that only one of the daughter cells was tracked after mitosis.
MASTL kinase assay
His-tagged ENSA was prepared as described2. Interphase and mitotic MASTL were immunoprecipitated with anti-FLAG agarose from synchronized HeLa cells expressing FLAG-EGFP-MASTL. The immunoprecipitates were then incubated with His-tagged ENSA in the buffer 80 mM Na β-glycerophosphate, 20 mM EGTA, 15 mM MgOAc, 10 mM MnCl2, 1 mM DDT pH 7.4 supplemented with 30 μM ATP and 5% v/v32 P-γ-ATP at 25 °C for 30 min room temperature. The reaction was stopped by addition of SDS-sample buffer, applied onto SDS-PAGE and analyzed with a PhosphorImager.
Antibodies and immunological methods
Antibodies against β-actin, FLAG, GFP (Sigma-Aldrich), CDC20, phosphor-histone H3Ser10 (Santa Cruz Biotechnology), phosphor-CDK1Tyr15, CDC27, CHK2 (BD Transduction Laboratories, Franklin Lakes, NJ, USA), CDH1 (Thermo Fisher Scientific, Waltham, MA, USA), phosphor-CHK2Thr68 (Calbiochem, San Diego, CA, USA), and ENSA (also recognize ARPP19) (Abcam, Cambridge, UK) were obtained from the indicated suppliers. Antibodies against CDK1 and cyclin B1 were gifts from Julian Gannon (Cancer Research UK). Rabbit polyclonal antibodies against MASTL were raised against bacterially expressed GST-MASTLC∆310 and then purified with a method involving membranes loaded with purified GST-MASTLC∆310 28. Immunoblotting and immunoprecipitation were performed as described previously25. For immunostaining of 53BP1, cells were washed twice with ice-cold PBS and fixed with methanol at −20 °C for 10 min followed by blocking with 3% BSA in 0.2% Tween-20 in PBS at 25 °C for 30 min. The cells were then incubated with anti-53BP1 antibody (H-300; Santa Cruz Biotechnology) at 4 °C for overnight. The samples were then incubated with Alexa Fluor 568 conjugated anti-rabbit IgG antibody (Life Technologies) at 25 °C for 2 h. The cells were counterstained with Hoechst 33342 for 1 min and viewed under fluorescence microscope using a 40X objective.
Statistical analysis
Box-and-whisker plots were generated using RStudio (Boston, MA , USA). Student’s t-test was used to calculate statistical significance (*P < 0.05; **P < 0.01; ***P < 0.001).
Additional Information
How to cite this article: Wong, P. Y. et al. MASTL(Greatwall) regulates DNA damage responses by coordinating mitotic entry after checkpoint recovery and APC/C activation. Sci. Rep. 6, 22230; doi: 10.1038/srep22230 (2016).
Supplementary Material
Acknowledgments
We thank Wing Yu Man and Chuan Shan for initial characterization of MASTL. We also thank Joyce P.Y. Mak for generating the FUCCI-expressing U2OS cells. This work was supported in part by the Research Grants Council grants 662213 and T13-607/12R to R.Y.C.P.
Footnotes
Author Contributions P.Y.W., H.T.M. and R.Y.C.P. conceived the project and designed experiments. P.Y.W., H.T.M. and H.J.L. carried out experiments. All authors contributed to writing the manuscript.
References
- Gharbi-Ayachi A. et al. The Substrate of Greatwall Kinase, Arpp19, Controls Mitosis by Inhibiting Protein Phosphatase 2A. Science 330, 1673–1677 (2010). [DOI] [PubMed] [Google Scholar]
- Mochida S., Maslen S. L., Skehel M. & Hunt T. Greatwall Phosphorylates an Inhibitor of Protein Phosphatase 2A That Is Essential for Mitosis. Science 330, 1670–1673 (2010). [DOI] [PubMed] [Google Scholar]
- Burgess A. et al. Loss of human Greatwall results in G2 arrest and multiple mitotic defects due to deregulation of the cyclin B-Cdc2/PP2A balance. Proc Natl Acad Sci USA 107, 12564–12569 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigneron S. et al. Greatwall maintains mitosis through regulation of PP2A. EMBO J 28, 2786–2793 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castilho P. V., Williams B. C., Mochida S., Zhao Y. & Goldberg M. L. The M phase kinase Greatwall (Gwl) promotes inactivation of PP2A/B55delta, a phosphatase directed against CDK phosphosites. Mol Biol Cell 20, 4777–4789 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J., Zhao Y., Li Z., Galas S. & Goldberg M. L. Greatwall kinase participates in the Cdc2 autoregulatory loop in Xenopus egg extracts. Mol Cell 22, 83–91 (2006). [DOI] [PubMed] [Google Scholar]
- Zhao Y. et al. Roles of Greatwall kinase in the regulation of cdc25 phosphatase. Mol Biol Cell 19, 1317–1327 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Fernandez M. et al. Greatwall is essential to prevent mitotic collapse after nuclear envelope breakdown in mammals. Proc Natl Acad Sci USA 110, 17374–17379 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastan M. B. & Bartek J. Cell-cycle checkpoints and cancer. Nature 432, 316–323 (2004). [DOI] [PubMed] [Google Scholar]
- Smith J., Tho L. M., Xu N. & Gillespie D. A. The ATM-Chk2 and ATR-Chk1 pathways in DNA damage signaling and cancer. Adv Cancer Res. 108, 73–112 (2010). [DOI] [PubMed] [Google Scholar]
- Chen Y. & Poon R. Y. The multiple checkpoint functions of CHK1 and CHK2 in maintenance of genome stability. Front Biosci. 13, 5016–5029 (2008). [DOI] [PubMed] [Google Scholar]
- Peng A., Yamamoto T. M., Goldberg M. L. & Maller J. L. A novel role for greatwall kinase in recovery from DNA damage. Cell Cycle 9, 4364–4369 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng A., Wang L. & Fisher L. A. Greatwall and Polo-like kinase 1 coordinate to promote checkpoint recovery. J Biol Chem. 286, 28996–29004 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archambault V., Zhao X., White-Cooper H., Carpenter A. T. & Glover D. M. Mutations in Drosophila Greatwall/Scant reveal its roles in mitosis and meiosis and interdependence with Polo kinase. PLoS Genet 3, e200 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T. M., Wang L., Fisher L. A., Eckerdt F. D. & Peng A. Regulation of Greatwall kinase by protein stabilization and nuclear localization. Cell Cycle 13, 3565–3575 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson R. & Eastman A. The cancer therapeutic potential of Chk1 inhibitors: how mechanistic studies impact on clinical trial design. Br J Clin Pharmacol. 76, 358–369 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover D. M. The overlooked greatwall: a new perspective on mitotic control. Open Biol. 2, 120023 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H. T., Tsang Y. H., Marxer M. & Poon R. Y. Cyclin A2-cyclin-dependent kinase 2 cooperates with the PLK1-SCFbeta-TrCP1-EMI1-anaphase-promoting complex/cyclosome axis to promote genome reduplication in the absence of mitosis. Mol Cell Biol. 29, 6500–6514 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaue-Sawano A. et al. Visualizing spatiotemporal dynamics of multicellular cell-cycle progression. Cell 132, 487–498 (2008). [DOI] [PubMed] [Google Scholar]
- Yu H. Cdc20: a WD40 activator for a cell cycle degradation machine. Mol Cell 27, 3–16 (2007). [DOI] [PubMed] [Google Scholar]
- Nagel R. et al. Genome-wide siRNA Screen Identifies the Radiosensitizing Effect of Downregulation of MASTL and FOXM1 in NSCLC. Mol Cancer Ther. 14, 1434–1444 (2015). [DOI] [PubMed] [Google Scholar]
- Yam C. H., Siu W. Y., Lau A. & Poon R. Y. Degradation of cyclin A does not require its phosphorylation by CDC2 and cyclin-dependent kinase 2. J Biol Chem. 275, 3158–3167 (2000). [DOI] [PubMed] [Google Scholar]
- Chan Y. W. et al. CDK1 inhibitors antagonize the immediate apoptosis triggered by spindle disruption but promote apoptosis following the subsequent rereplication and abnormal mitosis. Cell Cycle 7, 1449–1461 (2008). [DOI] [PubMed] [Google Scholar]
- Tsang Y. H., Han X., Man W. Y., Lee N. & Poon R. Y. Novel functions of the phosphatase SHP2 in the DNA replication and damage checkpoints. PLoS One 7, e49943 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon R. Y., Toyoshima H. & Hunter T. Redistribution of the CDK inhibitor p27 between different cyclin.CDK complexes in the mouse fibroblast cell cycle and in cells arrested with lovastatin or ultraviolet irradiation. Mol Biol Cell 6, 1197–1213 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H. T. & Poon R. Y. Synchronization of HeLa cells. Methods Mol Biol. 761, 151–161 (2011). [DOI] [PubMed] [Google Scholar]
- Siu W. Y., Arooz T. & Poon R. Y. Differential responses of proliferating versus quiescent cells to adriamycin. Exp Cell Res. 250, 131–141 (1999). [DOI] [PubMed] [Google Scholar]
- Ritter K. Affinity purification of antibodies from sera using polyvinylidenedifluoride (PVDF) membranes as coupling matrices for antigens presented by autoantibodies to triosephosphate isomerase. J Immunol Methods 137, 209–215 (1991). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.