Abstract
Epstein–Barr virus (EBV) was used to immortalize human peripheral B lymphocytes committed to the production of autoantibodies in healthy subjects and in patients with various autoimmune diseases. EBV-transformed B lymphocytes producing human monoclonal autoantibodies were fused with the human–mouse heterohybridoma F3B6 cells to stabilize the production of monoclonal antibodies (mAb). The present method of generating human B cell clones producing mAbs now makes it possible to analyze in detail the nature of human autoantibodies with respect to antigen-binding specificity, affinity, corresponding autoepitopes, and the variable (V) region structure.
It has been proposed that aggression of the self is prevented by deletion of the B lymphocytes committed to the production of self-reactive antibodies (1). This, however, contrasts with the recent evidence that B cells capable of producing antibodies binding to self-antigens can be detected not only in patients with various autoimmune diseases but also in healthy subjects (2–8). This was first demonstrated by the affinity chromatography isolation of natural antibodies that react with various self- and exogenous antigens from sera of healthy subjects (7) or from sera containing monoclonal Ig (8). However, the precise characterization of these natural antibodies was not performed at the clonal level until techniques for generating human mAbs became available (4, 5, 9, 10). In the present paper, we describe the methodological approach that we utilized to generate human mAbs to self- and exogenous antigens from peripheral B lymphocytes of both healthy subjects and patients with various autoimmune diseases. Epstein–Barr virus (EBV) is used to immortalize human peripheral B lymphocytes. The EBV-transformed B lymphocytes producing antibodies to various self- and exogenous antigens are fused with F3B6 cells, an Ig nonsecretor human–mouse fusion partner. The resulting EBV-transformed B cell hybrids display a clonability and mAb secreting rate much higher than those of the parental EBV-transformed B cells. Formal cloning of these cell hybrids yields mAb-producing cell lines suitable for fine immunological analysis of mAbs and for cloning and sequencing of the genes encoding the antigen-binding site variable (V) regions.
MATERIALS
Equipment
Biological safety cabinet, Model 237001, class 2, type A, Forma Scientific (Marietta, OH)
Table-top refrigerated centrifuge, Model TJ-6, Beckman Instruments Inc. (Columbia, MD)
Table-top centrifuge, IEC, No. 20671-009, VWR (Piscataway, NJ)
Water-jacketed incubator, Model 3158, Forma Scientific
Electric pipet-Aid, No. 13-681-15, Fisher Scientific (Springfield, NJ)
Microscope, Leitz Laborlux, Type 020-435.037, Kraner Scientific (Yonkers, NY)
Microscope, inverted tissue culture model, Carl Zeiss, No. 471281, Baltimore Instrument Co., Inc (Baltimore, MD)
Water bath, Model 185, No. 9825h36, Thomas Scientific (Swedesboro, NJ)
Chemicals and Reagents
RPMI 1640, No. 320-1870AJ, GIBCO (Grand Island, NY)
Fetal bovine serum (FBS), No. 240-1870AJ, GIBCO
L-Glutamine, No. 320-5030AG, GIBCO
Antibiotic-antimycotic solution 100X, No. 600-5240AG, GIBCO
Phosphate-buffered saline (PBS), No. 310-4040AJ, GIBCO
AET (2-aminoethylisothiouronium bromide hydrobromide), No. A5879, Sigma (St. Louis, MO)
Lymphocyte separating medium (LSM), No. 50494, Organon-Teknica-Cappel (Durham, NC)
Polyethylene glycol (PEG), molecular weight 3000–3700, No. P2906, Sigma
Sodium pyruvate, No. S8636, Sigma
Oubain, No. 03125, Sigma
Hypoxanthine, No. H9377, Sigma
Azaserine, No. A4142, Sigma
Dimethyl sulfoxide, No. BP231-1000, Fisher Scientific
L-Leucine methyl ester, No. L9000, Sigma
Media and Solutions
-
FBS–RPMI
Mix 500 ml of RPMI 1640, 50 ml of FBS, 5 ml of antibiotic-antimycotic solution, 5 ml of L-glutamine.
-
Selection medium
Mix FBS–RPMI containing 1% sodium pyruvate, 10−6 M oubain, 10−4 M hypoxanthine, 1 μg/ml azaserine.
-
AET solution
Dissolve 0.8 g of AET in 10 ml of distilled water. Adjust pH to 8.0 with 5 N NaOH. Filter sterilize.
-
L-Leucine methyl ester solution
Weigh 90.7 mg of L-leucine methyl ester and dissolve in 10 ml of RPMI 1640 containing L-glutamine and antibiotic–antimycotic solution. Add 30 μl of 5 M NaOH. Filter sterilize.
-
50% PEG solution
Autoclave 5 g of PEG and add RPMI 1640 to 10 ml. Aliquot and keep away from light.
Supplies
1-, 5-, 10-, and 25-ml pipets, Falcon, Nos. 53300-283, -421, -523, and -567, Fisher Scientific
50-ml centrifuge tubes, Falcon, No. 14-959-11B, Fisher Scientific
96-well round-bottom plate, Corning, No. 08-757-153, Fisher Scientific
96-well flat-bottom plate, Corning, No. 08-757-155, Fisher Scientific
24-well tissue culture plate, Corning, No. 08-757-156, Fisher Scientific
25-, 75-, and 150-cm2 tissue culture flasks, Corning, Nos. 10-126-39, -41, and -33, Fisher Scientific
Cells
B95-8 cells (EBV-transformed marmoset leukocytes), ATCC CRL 1612
F3B6 cells (mouse–human hybrid), ATCC HB 8785
Sheep red blood cells (SRBC), No. CS-1114, Colorado Serum Co. (Denver, CO)
PROCEDURE
A. Preparation of EBV
Culture marmoset lymphoma B95-8 cells in 75 ml of FBS–RPMI under a 5% CO2 atmosphere at 37°C in 150-cm2 culture flasks to confluency.
Add 75 ml of fresh FBS–RPMI to each flask and incubate confluent cultures for 3 to 5 more days.
Collect culture fluid.
Clear it of debris by centrifugation at 1500g for 45 min at 4°C.
Make 1-ml aliquots and store them at −80°C.
Thaw one aliquot of EBV-containing fluid for titration. Virus preparation should contain at least 5×106 transforming U/ml (11).
B. Preparation of AET-Treated SRBC
Wash packed SRBCs with PBS three times.
Incubate SRBCs in AET solution for 30 min at 37°C.
Wash SRBCs with ice-cold PBS five times.
C. Preparation of B Cells
Add an equal volume of PBS to freshly drawn blood or buffy coat.
Apply 30 ml of prediluted blood or buffy coat to 20 ml of LSM in 50-ml centrifuge tubes.
Centrifuge at 400g for 20 min at room temperature.
Collect peripheral blood mononuclear cells (PBMC) from the interface between plasma and LSM.
Gently wash PBMC with RPMI 1640.
Suspend washed PBMC in an appropriate volume of L-leucine methyl ester solution to a density of 5×106/ml.
Incubate for 40 min at room temperature (vortex gently every 10 min).
Centrifuge PBMC at 400g for 10 min.
Wash PBMC twice with Hanks’ balanced salt solution.
Resuspend PBMC in FBS–RPMI.
Mix PBMC with AET-treated SRBC at a ratio of about 1:100 and incubate on ice for 2 h.
Check for rosette formation by observing cells under a microscope.
Apply the PBMC–SRBC mixture to LSM in 50-ml tubes and centrifuge at 500g for 45 min at room temperature.
Collect PBMC from interface between LSM and medium by aspiration, and wash with PBS three times.
Resuspend PBMC in 10 ml of PBS and count. In general, 100 ml of fresh blood yields 108 PBMC and 107 B cells.
D. Preparation of Feeder Cells
Follow steps 1 to 5 in procedure C.
Irradiate PBMC at 1800 rad.
Wash PBMC twice with RPMI 1640.
Resuspend PBMC in 10 ml of RPMI 1640 and count.
Resuspend PBMC at a density of 2×106/ml. Plate 100 μl of PBMC suspension in each well of a 96-well round-bottom microculture plate. Alternatively, resuspend PBMC at a density of 4×106/ml, and plate 100 μl of this suspension in each well of a 96-well flat-bottom microculture plate.
E. Infection of B Lymphocytes by EBV and Detection of B Cells Producing Antibodies with a Given Specificity and/or Class
Resuspend 5×107 enriched B cells in a freshly thawed 1.0-ml EBV aliquot and incubate for 1 h at 37°C.
Resuspend cells in FBS–RPMI to a density of 5×104/ml.
Incubate cells under a 5% CO2 atmosphere at 37°C overnight.
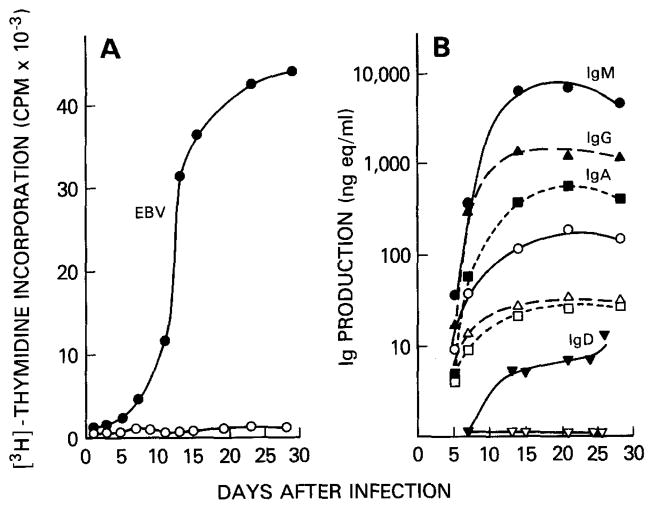
Distribute 200, 100, 40, 20, 10, 5, 2.5, and 1.25 B cells per well in 96-well round-bottom plates containing feeder cells. After EBV infection, B cells proliferate in culture for several weeks and produce Ig. The results of a typical B cell proliferation and Ig production experiment after infection by EBV are shown Fig. 1.
Incubate microcultures under a 5% CO2 atmosphere at 37°C for 10 days.
Discard 100 μl of supernatant from each well, add 100 μl of fresh FBS–RPMI, and continue incubation for another 4 days.
Harvest 50 μl of supernatant and analyze it for the content of antibodies with a given specificity using antigen-specific assays, such as ELISA or fluorescence antibody staining. In each assay, the specificity of the anti-Ig probe used should be chosen according to the targeted Ig class.
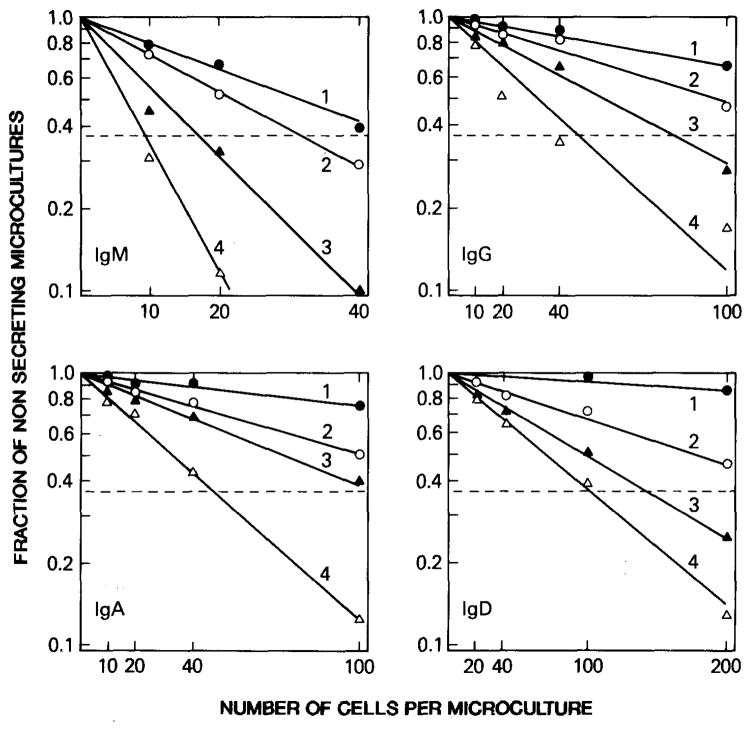
The frequency of B cell clones producing antibody with a given specificity and/or class can be calculated using Poisson distribution analysis as described (5,11). Similarly, Poisson distribution analysis can be used to determine the frequency of B cells committed to the production of Ig of different classes. The results of a typical experiment designed to determine the frequency of the peripheral blood B cells committed to the production of IgM, IgG, IgA, and IgD are shown in Fig. 2.
FIG. 1.
EBV-induced proliferation and Ig production in human peripheral blood B cells by EBV. To measure proliferation and antibody production rate, EBV-infected (closed symbols) or mock-infected (open symbols) B cells from a single donor were distributed at a density of 1×105/well in microculture wells containing irradiated PBMC as feeder cells. (A) The 24-h[3H]thymidine incorporation; (B) IgM, IgG, IgA, and IgD concentrations in the culture supernatants.
FIG. 2.
Enumeration of IgM-, IgG-, IgA-, and IgD-producing lymphocytes among EBV-infected B cells. B cells from one subject were infected with EBV and then distributed at varying numbers in microculture wells. After 1 (solid circles), 2 (open circles), 3 (solid triangles), and 4 (open triangles) weeks, the supernatants were tested for their IgM, IgG, IgA, and IgD contents. The fraction of negative wells (wells not containing Ig) of the total wells seeded at each cell concentration was plotted against the number of cells plated per well. Frequency of Ig-secreting cells was calculated using Poisson distribution analysis. The projection on the X axis of the intercept of (---) (fraction of negative wells, 0.37) with each line derived from the experimental data corresponds to the cell dose containing one B lymphocyte producing IgM, IgG, IgA, or IgD.
F. Establishment of EBV-Transformed B Cell Lines
Transfer the cells from a microculture well(s) that contains antibodies of the desired specificity and/or class into a new well of a 96-well round-bottom microculture plate without feeder cells.
Culture cells for 2 to 3 days and transfer them into a well of a 96-well flat-bottom microculture plate without feeder cells.
As the cells grow, adjust the volume of the culture so that cell density is always between 2×105 and 1×106/ml.
Test culture fluids for production of antibody.
Distribute the EBV-transformed B cells from a positive culture at 50, 25, 10, 5, 2.5, 1.0, and 0.5 cells/well in 96-well round-bottom microculture plates containing feeder cells.
Incubate subcultures for 10 to 14 days at 37°C.
Test supernatant for antibody production as described.
Repeat steps 1 to 7 twice to yield monoclonal cell lines.
G. Somatic Cell Hybridization
Prepare 1×107 EBV-transformed B cells producing antibody of desired specificity and/or class.
Prepare an equal number of F3B6 myeloma cells in culture.
Mix EBV-transformed B cells with F3B6 myeloma cells in 50 ml of prewarmed RPMI 1640.
Centrifuge for 5 min at 400g.
Discard supernatant.
Repeat steps 3 to 5 twice.
Loosen pellet in a 37°C water bath for 5 min with intermittent shaking.
Add prewarmed 50% PEG to a final concentration of 40%.
Incubate for 4 min at 37°C in water bath.
Add 50 ml of prewarmed RPMI 1640.
Centrifuge at 400g for 5 min.
Add 10 ml of prewarmed selective medium and incubate for 1 h at 37°C.
Resuspend the cells to 1×106 ml in selective medium and distribute 200 μl per well in 96-well flat-bottom plates containing feeder cells.
Incubate under a 5% CO2 atmosphere at 37°C.
Change the medium on Days 1, 3, and 7.
On Day 8, take 50 μl of supernatant for detection of antibodies of desired specificity and/or class.
Transfer the cell hybrids producing antibodies of desired specificity and/or class into another well of a 96-well flat-bottom microculture plate without feeder cells, and culture them in FBS–RPMI.
Increase the culture volume as cells grow.
Distribute the cell hybrids at 2, 1, and 0.5 cell/well in 96-well flat-bottom microculture plates containing feeder cells.
Culture for 10 days.
Harvest 50 μl of the supernatant for detection of antibodies of desired specificity and/or class.
Repeat steps 17 to 21 using cell hybrids positive for specific antibody production until all the cell hybrids growing in the microculture wells seeded with 0.5 cell/well yield antibodies of desired specificity.
DISCUSSION
The unique advantage of EBV as a tool for analyzing the human B cell repertoire is that it serves as both a potent B-cell-activating stimulus and an efficient transforming agent (12–26). Two sequential, discrete events take place after incubation of B lymphocytes with EBV. First, EBV attaches to the cell surface CR2, which is present on all mature B cells and late pre-B cells, but not on actively proliferating B lymphocytes in S phase (11). In most cases, subsequent virus internalization leads to infection of the cells, as revealed by the expression of EBV-related antigens, and then to cell activation. Second, after viral infection, actual cell transformation may ensue, yielding cell blasts capable of continuous proliferation and steady Ig secretion (Fig. 1).
EBV is equally efficient in activating and transforming B cells bearing surface μ, γ, and α Ig heavy chains to produce IgM, IgG, and IgA, respectively. Indeed, we recently showed that the percentage of peripheral blood B cells bearing surface μ, γ, and α IgH chains is similar to the percentage of cells producing IgM, IgG, and IgA, respectively, after EBV infection (5). Moreover, no significant rate of isotype switch is induced by EBV infection (Table 1). EBV can also activate and transform different B cell subsets, for example, CD5+ and CD5− B cells, with similar efficiency (3, 4). Thus, the strong, efficient, and balanced B cell activation induced by EBV is instrumental in investigating the clonal diversity of the human B cell repertoire (5, 26). Indeed, the use of EBV in conjunction with limiting dilution assay made it possible to enumerate the frequency of B cells committed to the production of the antibodies to a variety of self- and exogenous antigens (12–26).
TABLE 1.
Immunoglobulin Class Produced by Surface Isotype-Selected and EBV-Transformed B Lymphocytes
| Surface markers for B cell selection | Percentage of B cells producing
|
Percentage of B cells immortalized | ||
|---|---|---|---|---|
| IgG | IgA | IgM | ||
| B1 (CD20) | 5.4 ± 1.5 | 5.4 ± 1.0 | 86.3 ± 4.1 | 2.1 ± 0.3 |
| γ heavy chains | 99.6 ± 0.7 | 0.1 + 0.2 | 0.3 ± 0.3 | 3.3 ± 0.4 |
| α heavy chains | 0.5 ± 0.3 | 98.6 + 0.9 | 0.9 ± 0.4 | 2.8 ± 0.3 |
| μ heavy chains | 0.5 ± 0.2 | 0.3 + 0.2 | 99.6 ± 0.8 | 4.0 ± 0.5 |
Note. Mononuclear cells were obtained from healthy subjects (NIH Blood Bank, Leukopheresis Research Program, NIH, Bethesda, MD) and depleted of monocytes and T lymphocytes. The residual enriched B lymphocyte fraction contained approximately 60% B cells. 2 × 107 enriched B cells (5 × 106/ml) from three different subjects were incubated for 1 h in ice-chilled sterile Hanks’ balanced salt solution without Ca2+ and Mg2+, without phenol red, with 1% bovine serum albumin (BSA–HBSS), and containing appropriate amounts FITC mouse monoclonal antibody to CD20 or of FITC goat F(ab′)2 fragment to human γ, α, or μ Ig heavy chains. A negative control sample of similar B cells (106) from each subject was simultaneously incubated with BSA–HBSS containing FITC goat F(ab′)2 fragment with irrelevant binding activity and allowed to react under similar conditions. After washing with cold BSA–HBSS, cells from all samples were resuspended at 2×106/ml in the same medium and applied at different times to a Model 440 FACS equipped with an argon laser (Becton and Dickinson Immunocytometry Systems, Mountain View, CA). Cells that had been sorted for γ, α, or μ Ig were then infected with EBV and cultured at 1000, 500,100, 50, 20, 10, 5, and 2.5 cells/well in 96-well plates in the presence of 105 irradiated (1800 rad) peripheral blood mononuclear cells as feeders. After 4 weeks, culture fluids were assayed for total Ig content and specific classes (IgG, IgA, and IgM) of Ig using ELISA. Standard reference curves were constructed using purified IgG, IgA, or IgM of known concentration. Wells containing more than 10 ng/ml of IgM, IgG, or IgA were scored as positive. Twenty-five to thirty percent of B cells that had been sorted for surface CD20 or specific Ig heavy chain isotype produced Ig after EBV infection. The proportion of lymphocytes producing Ig of various classes is expressed as a percentage of total Ig-producing B cells. Numbers are mean values ± standard deviation of three experiments using lymphocytes from different donors. Fraction of B lymphocytes producing Ig was calculated by limiting dilution methodology and analysis according to Poisson distribution. Forty-eight microculture wells were used for each cell dose. The frequency of immortalized B lymphocytes was determined by limiting dilution and analysis based on Poisson distribution after 6 weeks of culture.
Using this system, we determined that a relatively large proportion (3 to 15%) of precursors of IgM-producing cells in the normal B cell repertoire are committed to the production of antibodies binding not only to exogenous antigens but also to self-antigens such as IgG Fc fragment, ssDNA, thyroglobulin, or insulin. These polyreactive autoantibodies were found to be produced by a discrete B cell subset, CD5+ B cells (3, 4). These findings argue against any hypothesis of clonal abortion of self-reactive B cells and provide an explanation for high numbers of self-reactive B cells capable of producing autoantibodies in healthy subjects (5). Although the B cells producing IgG or IgA antibodies to self-antigens can be detected in healthy subjects, the frequency of these B cells is very low (less than 0.037 and 0.087% of total IgG- and IgA-producing cells, respectively) and the IgG or IgA autoantibodies produced by these B cells are polyreactive (5). In contrast, we could readily identify precursors of cells committed to the production of monoreactive autoantibodies in various autoimmune diseases but not in healthy subjects. For example, in SLE patients, the B cells committed to the production of IgG antibodies to ssDNA are approximately 0.5% of the total IgG-producing cells, that is, at least 15-fold more frequent than those in healthy subjects. Similarly, in Hashimoto disease patients, B cells committed to the production of IgG to thyroglobulin or thyroid peroxidase are approximately 20-fold more frequent than those in healthy subjects (4, 5). Thus, the expansion of the B cells producing monoreactive IgG autoantibodies seems to be a characteristic of patients with autoimmune diseases.
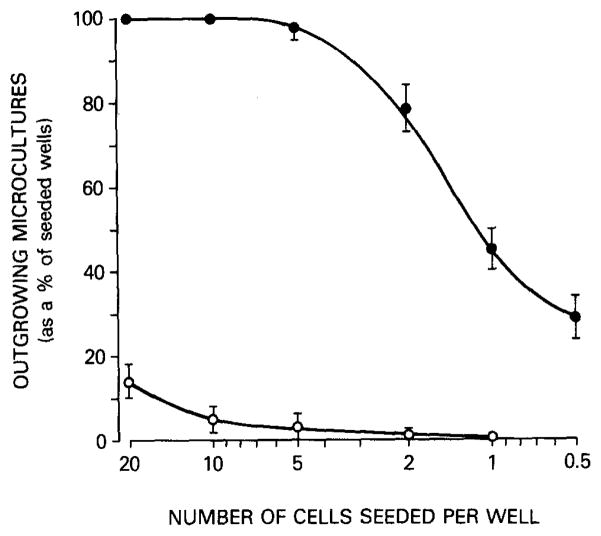
EBV-transformed B cells can be used as a source to generate human mAbs. However, they can be cloned only with difficulty at a low density (less than 5 cells/well). Their growth and antibody production are sometimes erratic when they are cultured beyond a short period (3 to 4 weeks). Therefore, the fusion of EBV-transformed B cells with a preconstructed Ig nonsecretor human–mouse fusion partner (4, 5, 21) or the introduction of an activated Ha-ras oncogene into EBV-transformed B cells (27) is crucial to overcoming these limitations. The resulting human–human–mouse hybrid clones display much higher plating efficiency, stability, and antibody secretion rate than their parental EBV-transformed B cells in long-term culture (Fig. 3). In addition, infection of B cells with EBV not only provides an essential clonal amplification step of B cells with desired antigen-binding specificity but also results in the formation of blasts that fuse much more efficiency than small resting B cells.
FIG. 3.
Plating efficiency of six EBV-transformed hybrid cells (closed circles) and their parental EBV-transformed cells (open circles). Six different EBV-transformed B cell clones were generated by three sequential clonings at 5 to 20 cells/well. These clones were then expanded and separately fused with F3B6 heteromyeloma cells. The resulting hybrids were subcultured at 0.5 cell/well and clones were generated. Parental EBV-transformed B cells and EBV-transformed hybrid cells were then seeded at 20, 10, 5, 2, 1, and 0.5/well in 96-well plates containing 105 irradiated peripheral blood mononuclear cells as feeders. The frequency of growing cultures was determined 2 and 6 weeks later for EBV-transformed hybrid cells and parental EBV-transformed B cells, respectively. Vertical bars represent the mean value ± standard deviation of the number of outgrowing cultures (as a percentage of seeded wells) for the six EBV-transformed hybrid cells and parental EBV-transformed B cells.
Using these methods we generated a number of human monoclonal autoantibodies from the peripheral blood B lymphocytes of patients with various autoimmune diseases, such as rheumatoid arthritis, insulin-dependent diabetes mellitus, Hashimoto disease, SLE, and primary biliary cirrhosis, and of healthy subjects (4–6, 12–26). As depicted in Table 2, two distinct populations of autoantibodies were generated from normal and autoimmune B cell reperoires.
TABLE 2.
Dissociation Constants of Human Polyreactive and Monoreactive Monoclonal Antibodies for Different Antigens
| Clone |
mAb
|
Donora | Selecting antigen | Pattern of reactivity | Antigenb
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Heavy chain | Light chain | Fc fragment of IgG | Insulin | Thyroglobulin | ssDNA | Tetanus toxoid | ||||
| Ab35 | μ | λ | HS | Fc fragment | Polyreactive | 6.0×10−5 | 1.0×10−7 | ndc | 4.2×10−7 | 2.0×10−7 |
| Ab40 | μ | λ | HS | Fc fragment | Polyreactive | 2.5×10−5 | 4.1×10−7 | nd | 1.7×10−7 | 1.0×10−7 |
| Ab43 | μ | λ | HS | Fc fragment | Polyreactive | 7.0×10−5 | 1.0×10−7 | 8.2×10−6 | 5.0×10−7 | 5.1×10−8 |
| P32.10.F1 | γ1 | κ | HS | Insulin | Polyreactive | nd | 3.3×10−6 | 5.5×10−7 | 1.0×10−6 | 4.0×10−7 |
| 232.F68.1 | α1 | λ | HS | ssDNA | Polyreactive | nd | 1.2×10−5 | 5.0×10−3 | 2.0×10−7 | 3.3×10−6 |
| P118.F68.1 | γ3 | κ | TT-vacc HS | Tetanus toxoid | Monoreactive | nd | nild | nil | nil | 3.0×10−11 |
| P364.E.11 | γ1 | λ | TT-vacc HS | Tetanus toxoid | Monoreactive | nd | nil | nil | nil | 4.5×10−11 |
| CL91.5.F2 | γ1 | κ | Treated-IDDM | Insulin | Monoreactive | nd | 2.5×10−7 | nil | nil | nil |
| Ab48 | γ3 | κ | Newly-IDDM | Insulin | Monoreactive | nd | 8.0×10−7 | 1.0×10−5 | nil | nil |
| 274.RA.F4 | μ | κ | RA | Fc fragment | Monoreactive | 6.0×10−7 | nil | nil | nil | nil |
| 274.RA.F1 | α | κ | RA | Fc fragment | Monoreactive | 2.0×10−7 | nil | nil | nil | nil |
| P75.5.1 | γ1 | κ | HD | Thyroglobulin | Monoreactive | nd | nil | 1.0×10−9 | nil | nil |
| P88.3.3.2 | γ3 | κ | HD | Thyroglobulin | Monoreactive | nd | nil | 7.8×10−11 | nil | nil |
| 233.18 | μ | κ | SLE | ssDNA | Monoreactive | nd | nil | nil | 1.3×10−8 | nil |
| 399.18 | γ1 | κ | SLE | ssDNA | Monoreactive | nd | nil | nil | 2.0×10−8 | nil |
HS, healthy subject: TT-vacc HS, healthy subject vaccinated with tetanus toxoid; treated-IDDM, insulin-treated insulin-dependent diabetes mellitus patient; newly IDDM, newly diagnosed insulin-dependent diabetes mellitus patient before insulin treatment; RA, rheumatoid arthritis patient; HT, Hashimoto disease patient.
Kd is expressed in mol/liter
nd, not determined.
nil, Kd high to be calculated (low affinity)
The first population consists of polyreactive, mainly IgM autoantibodies with variable affinities (Kd, 10−5 to 10−8 mol/liter) for various self- and exogenous antigens. These polyreactive autoantibodies were demonstrated to be the exclusive product of CD5+ B cells of a normal B cell repertoire (4) and may be what has been referred to as the natural antibodies of the serum (7,8). Analysis of more than 30 human mAbs generated using B cells from healthy subjects revealed that each mAb bound not only to the antigen used for its selection but also to other antigens, in general with different efficiency (4–6, 23, 26). Moreover, binding of the polyreactive mAbs to each of the ligands in solid phase was inhibited in a dose-dependent fashion and with different efficiency not only by the soluble homologous ligand but also by soluble heterologous ligands (4–6, 23). Thus, each polyreactive mAb displays different binding activities for different antigens, and different mAbs display different binding activities for the same antigen, suggesting that, despite their broad reactivity, each polyreactive mAb possesses a discrete pattern of fine specificity. Parallel experiments involving equimolar amounts of a whole natural antibody molecule and its isolated cleaved fragments showed that the multiple antigen-binding function of polyreactive antibodies is mediated by Fab moiety (28). Because of their broad reactivity with a variety of microorganisms, it is possible that these antibodies play a major role in the primary line of defense against infections. In addition, owing to their ability to bind self-antigens, they may contribute to the establishment of autoimmune conditions, particularly those associated with significant expansion of the CD5+ B cell subpopulation, including rheumatoid arthritis (12, 29).
The second population consists of monoreactive, mainly IgG and IgA autoantibodies with high affinities (Kd, 10−7 to 10−11 mol/liter) for the disease-relevant antigens. Most of these autoantibodies, which were demonstrated to be mainly the product of CD5− B cells in SLE patients (13) and of CD5+ B cells in rheumatoid arthritis patients (12), can be generated using B cells from autoimmune patients but not those from healthy subjects. The affinities of these monoreactive autoantibodies are as high as those of the antibodies that are generated after the active immunization of healthy subjects with exogenous antigens, such as tetanus toxoid or rabies virus (5, 14), or generated from patients with chronic viral infections, such as human T cell leukemia virus type 1 (HTLV-1) infection (15, 20). These and other findings suggest that monoreactive and high-affinity autoantibodies are generated by an antigen-driven secondary immune response involving affinity maturation of the Ig VH and VL gene. Although polyclonal B cell activation has been proposed as a mechanism responsible for the production of autoantibodies in systemic autoimmune diseases such as SLE (16), the demonstration that autoantibodies to ssDNA are monoreactive and of high affinity suggests that a polyclonal B cell activation cannot be the only mechanism underlying the production of autoantibodies in SLE patients. A similar demonstration of the presence of monoreactive high-affinity autoantibodies to the relevant self-antigens in patients with rheumatoid arthritis, insulin-dependent diabetes mellitus, Hashimoto disease, and primary biliary cirrhosis also suggests that the antigen-driven process of B cell maturation is operative in these autoimmune diseases (4–6,12,13,19).
A central issue related to the understanding of the physiologic roles of polyreactive or natural antibodies is whether the polyreactive antibody-producing cell precursors can give rise to B cells producing monoreactive autoantibodies with high affinity. Our data, although limited in scope, show that in patients with two major systemic autoimmune diseases, such as SLE and rheumatoid arthritis, the specific autoantibodies share the same cellular origin (CD5+ B cells in rheumatoid arthritis and CD5+ and CD5− CD45RA10 B cells in SLE) and similar or identical Ig V genes or gene families with polyreactive antibodies from healthy subjects (17, 18, 22, 24). Consistent with our demonstration that B cells of the CD5+ “lineage” display an Ig hypermutation mechanism similar to that of “con” B cells, application of self or self-cross-reactive antigenic pressure to CD5+ and/or CD5− CD45RA10 B cell clones may result in generation of high-affinity somatically mutated autoantibodies (17, 18, 22, 24, 25, 29). These human monoclonal autoantibodies with high affinity and specificity have also been instrumental in identifying as-yet-unknown autoepitopes of self-antigens in various autoimmune diseases (19).
Footnotes
This work was supported in part by U.S. Public Health Service Grants AR-40908 and CA 16804. This is Publication 17 from The Jeanette Greenspan Laboratory for Cancer Research.
References
- 1.Burnet FM. The Clonal Selection Theory of Acquired Immunity. Cambridge Univ. Press; Cambridge: 1959. [Google Scholar]
- 2.Casali P, Inghirami G, Nakamura M, Davies TF, Notkins AL. Science. 1986;234:476–479. doi: 10.1126/science.3020687. [DOI] [PubMed] [Google Scholar]
- 3.Casali P, Burastero SE, Nakamura M, Inghirami G, Notkins AL. Science. 1987;236:77–81. doi: 10.1126/science.3105056. [DOI] [PubMed] [Google Scholar]
- 4.Nakamura M, Burastero SE, Notkins AL, Casali P. J Immunol. 1988;140:4180–4186. [PubMed] [Google Scholar]
- 5.Nakamura M, Burastero SE, Ueki Y, Larrick JW, Notkins AL, Casali P. J Immunol. 1988;141:4165–4172. [PubMed] [Google Scholar]
- 6.Casali P, Nakamura M, Ginsberg-Fellner F, Notkins AL. J Immunol. 1990;144:3741–3747. [PubMed] [Google Scholar]
- 7.Guilbert G, Dighiero G, Avrameas S. J Immunol. 1982;128:2779–2787. [PubMed] [Google Scholar]
- 8.Dighiero G, Guilbert B, Fermand JP, Lymberi P, Danon F, Avrameas S. Blood. 1983;62:264–270. [PubMed] [Google Scholar]
- 9.Shoenfeld Y, Hsu-Lin SC, Gabriels JE, Silberstein LE, Furie BC, Furie B, Stollar BD, Schwarts RS. J Clin Invest. 1982;70:205–208. doi: 10.1172/JCI110595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shoenfeld Y, Rauch J, Massicotte H, Datta SK, Andre-Schwarts J, Stollar BD, Schwarts RS. N Engl J Med. 1983;308:414–420. doi: 10.1056/NEJM198302243080802. [DOI] [PubMed] [Google Scholar]
- 11.Inghirami G, Nakamura M, Balow JE, Notkins AL, Casali P. J Virol. 1988;62:2453–2463. doi: 10.1128/jvi.62.7.2453-2463.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burastero SE, Casali P, Wilder RL, Notkins AL. J Exp Med. 1988;168:1979–1992. doi: 10.1084/jem.168.6.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Casali P, Burastero SE, Balow JE, Notkins AL. J Immunol. 1989;143:3476–3483. [PubMed] [Google Scholar]
- 14.Ueki Y, Goldfarb IS, Harindranath N, Gore M, Koprowski H, Notkins AL, Casali P. J Exp Med. 1990;171:19–34. doi: 10.1084/jem.171.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakamura M, Itoyama Y, Kuroki M, Nakano S, Kondo S, Nagafuchi S, Kira J, Ichinose I, Mitsugi K, Anzai K, Mori H, Fukui M, Okamura S, Niho Y. J Neuroimmunol. 1992;37:35–45. doi: 10.1016/0165-5728(92)90153-c. [DOI] [PubMed] [Google Scholar]
- 16.Klinman DM, Steinberg AD. J Exp Med. 1987;165:1755–1760. doi: 10.1084/jem.165.6.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sanz I, Casali P, Thomas W, Notkins AL, Capra DJ. J Immunol. 1989;142:4054–4061. [PubMed] [Google Scholar]
- 18.Harindranath N, Goldfarb IS, Ikematsu H, Burastero SE, Wilder RL, Notkins AL, Casali P. Int Immunol. 1991;3:865–875. doi: 10.1093/intimm/3.9.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matsui M, Nakamura M, Ishibashi H, Koike K, Kudo J, Niho Y. Hepatology. 1993 in press. [PubMed] [Google Scholar]
- 20.Kuroki M, Nakamura M, Itoyama Y, Tanaka Y, Shiraki H, Baba E, Esaki T, Tatsumoto T, Nagafuchi S, Nakano S, Niho Y. J Immunol. 1992;149:940–948. [PubMed] [Google Scholar]
- 21.Larrick JW, Chiang YL, Sheng-Dong R, Senyk G, Casali P. In: In Vitro Immunization in Hybridoma Technology. Borrebaek CAK, editor. Elsevier; Amsterdam: 1988. pp. 231–246. [Google Scholar]
- 22.Kasaian MT, Ikematsu H, Casali P. J Immunol. 1992;148:2690–2702. [PMC free article] [PubMed] [Google Scholar]
- 23.Casali P, Notkins AL. Immunol Today. 1989;10:364–368. doi: 10.1016/0167-5699(89)90268-5. [DOI] [PubMed] [Google Scholar]
- 24.Ikematsu H, Harindranath N, Ueki Y, Notkins AL, Casali P. J Immunol. 1993;150:1325. [PMC free article] [PubMed] [Google Scholar]
- 25.Ikematsu H, Nakamura M, Ginsberg-Fellner F, Schettino EW, Casali P. 1992 Submitted for publication. [Google Scholar]
- 26.Casali P, Notkins AL. Annu Rev Immunol. 1989;7:513–535. doi: 10.1146/annurev.iy.07.040189.002501. [DOI] [PubMed] [Google Scholar]
- 27.Shammah S, Mantovani TL, Dalla-Favera R, Casali P. J Immunol Methods. 1993 doi: 10.1016/0022-1759(93)90004-q. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Riboldi P, Kasaian MT, Mantovani L, Ikematsu H, Casali P. In: The Molecular Pathology of Autoimmunity. Bona CA, Siminovitch K, Zanetti M, Theophilopoulos AN, editors. Gordon & Breach; Philadelphia: 1993. [Google Scholar]
- 29.Mantovani L, Wilder RL, Casali P. J Immunol. 1993 in press. [PMC free article] [PubMed] [Google Scholar]