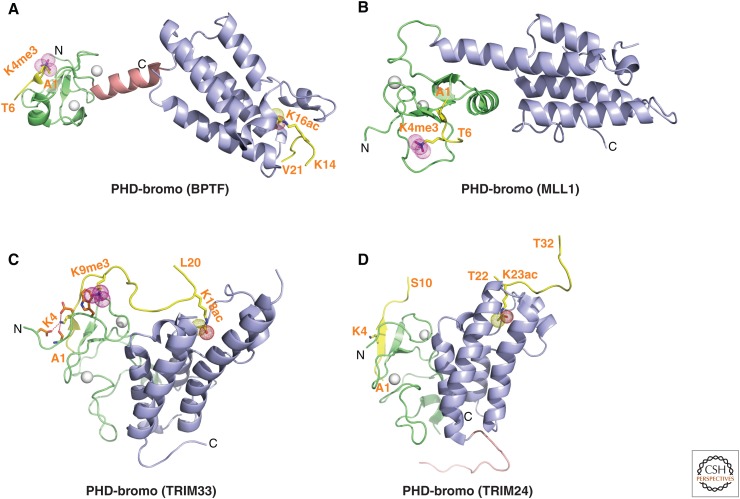
Figure 11.
Structures of PHD-bromo cassettes involved in multivalent readout. (A) 2.0-Å crystal structure of the PHD-bromo cassette of BPTF bound to H3(1-15)K4me3 peptide (PDB: 2F6Z). A separate 1.8-Å crystal structure of the BPTF bromodomain bound to H4(12-21)K16ac peptide was also solved (PDB: 3QZS) and that information was superpositioned on the structure shown in this panel. The bound H3(1-15)K4me3-containing peptide can be traced from A1 to T6, whereas the bound H4(12-21)K16ac-containing peptide can be traced from K14 to V21 in the complexes. (B) 1.9-Å crystal structure of the MLL1 PHD-bromo cassette bound to H3(1-9)K4me3 peptide (PDB: 3LQJ). The bound H3(1-9)K4me3-containing peptide can be traced from A1 to T6 in the complex. (C) 2.7-Å crystal structure of the TRIM33 PHD-bromo cassette bound to H3(1-22)K9me3K18ac peptide (PDB: 3U5O). The bound H3(1-22)K9me3K18ac peptide can be traced from A1 to L20 in the complex. (D) The crystal structures of the TRIM24 PHD-bromo cassette bound to H3(1-10) peptide (2.0 Å) (PDB: 3O37) and bound to H3(13-32)K23ac peptide (1.9 Å) (PDB: 3O37). The structures were superpositioned to generate the composite structure shown in this panel. The bound H3(13-32)K23ac peptide can only be traced from T22 to T32 in the complex.