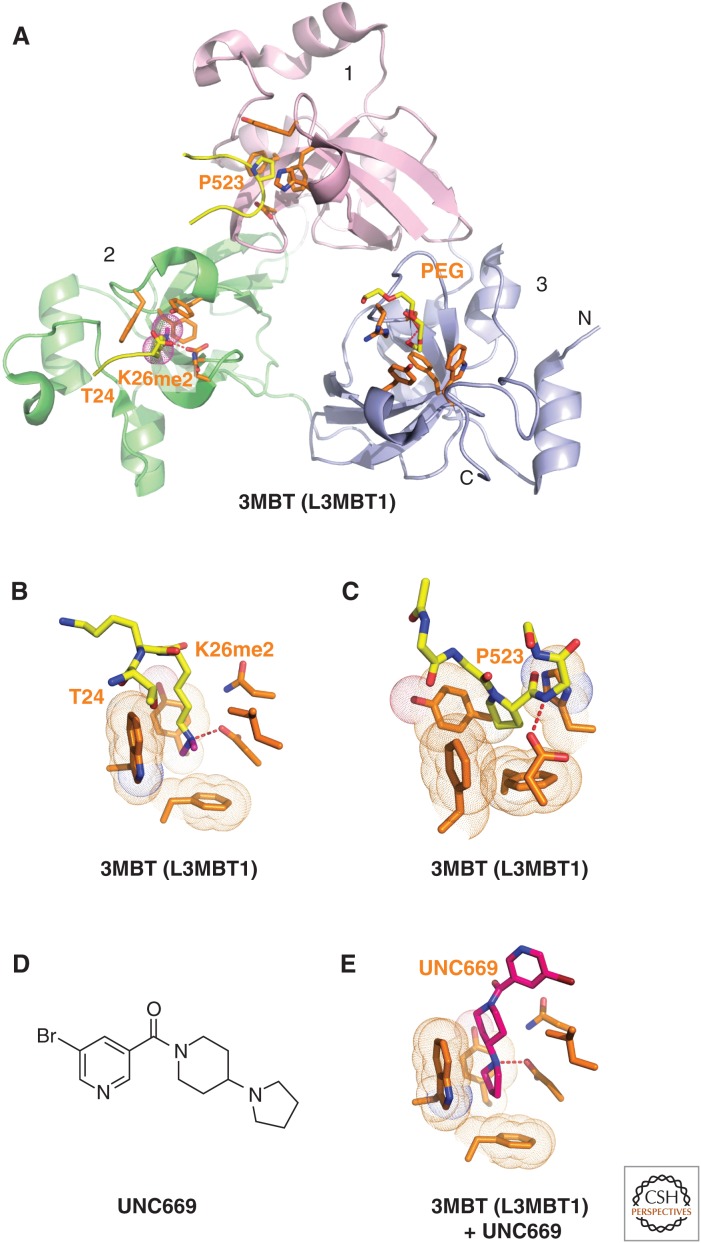
Figure 6.
Structures of L3MBTL1 bound to methylated lysine histone peptides and an inhibitor. (A) 1.66-Å crystal structure of the complex-containing L3MBTL1 bound to H1(22-26)K26me2 peptide (PDB: 2RHI). A carboxy-terminal peptide from an adjacent L3MBTL1 in the crystal lattice inserts Pro523 into the aromatic-lined pocket of MBT domain 1 (in pink). The dimethylammonium group of bound K26me2 inserts into the aromatic-lined pocket of MBT domain 2 (in green) with the K26me2-containing H1 peptide traced from T24 to K26me2. A polyethylene glycol (PEG) molecule inserts into the aromatic-lined pocket of MBT domain 3 (in blue). (B) Details of how the H1K26me2 dimethylammonium group inserts into the aromatic-lined pocket of MBT domain 2. (C) Structural detail showing how the proline from an adjacent L3MBTL1 in the crystal lattice inserts into the aromatic-lined pocket of MBT domain 1. This pocket is shallower than the one shown in B. (D) Chemical formula of UNC669. (E) Details of how UNC669 inserts into the aromatic-lined pocket of MBT domain 2, based on the 2.55-Å crystal structure of L3MBTL1 bound to UNC669 (PDB: 3P8H).